7.1 Forms of Energy
Christelle Sabatier and Hannah Nelson
Learning Objectives
By the end of this section, you will be able to do the following:
- Define “energy.”
- Explain the difference between kinetic and potential energy.
- Explain the concepts of free energy and activation energy.
- Describe endergonic and exergonic reactions.
Energy is defined as the ability to do work. As you’ve learned, energy exists in different forms. For example, electrical energy, light energy, and heat energy are all different types of energy. While these are all familiar types of energy that one can see or feel, there is another type of energy that is much less tangible. This energy is associated with something as simple as an object held above the ground. In order to appreciate the way energy flows into and out of biological systems, it is important to understand more about the different types of energy that exist in the physical world.
Potential Energy and Chemical Energy
Moving water, such as in a waterfall or a rapidly flowing river, has kinetic energy. Now does the motionless water trapped behind a dam have energy associated with it? The answer is yes. The water that is held back by the dam has energy associated with it that is fundamentally different from the kinetic energy of water in motion. This form of energy results from the fact that there is the potential for the water to do work. If it is released, indeed it would do work (Figure 7.1.1). Because this type of energy refers to the potential to do work, it is called potential energy.

Energy in Ion Gradients
In Chapter 4.4, we explored the transformation between potential and kinetic energy to drive work in our discussions of secondary active transport. As you recall, secondary active transport is when a transporter makes use of an existing ion gradient across the membrane (potential energy) to drive the movement. Consider the sodium/glucose co-transporter depicted in Figure 7.1.2.
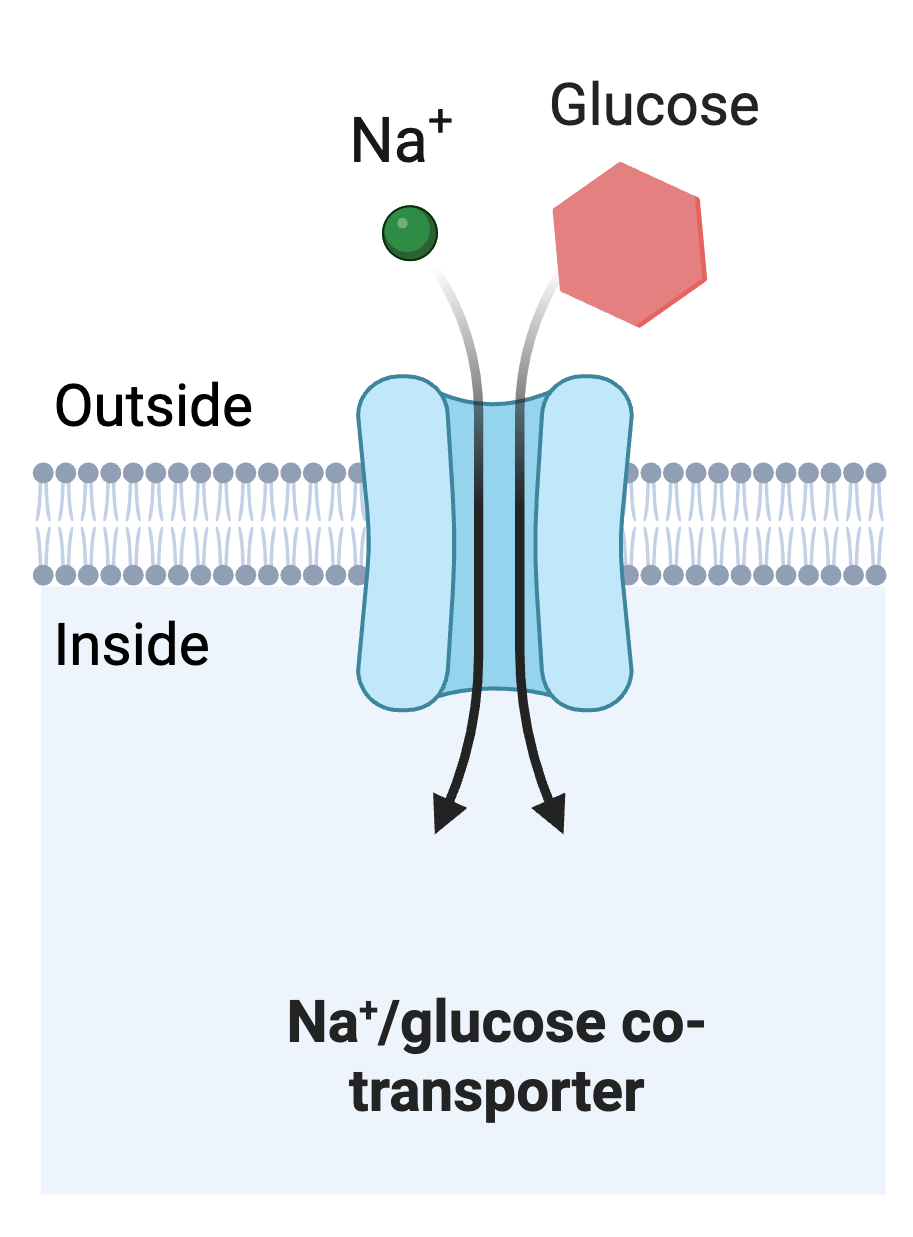
This co-transporter actively transports glucose from the outside of the cell to the inside of a cell against its concentration gradient. Meaning that as a result of the work of this co-transporter, the cell will have a higher concentration of glucose inside than outside. This work requires energy, which is derived from the movement of the sodium ion from outside the cell to inside the cell. In animal cells, sodium exists in a gradient across the cell membrane due to the actions of the sodium-potassium pump with a high concentration of sodium outside the cell and a relatively low concentration of sodium inside the cell. The work of the sodium-potassium pump sets up potential energy through this sodium gradient. The sodium-glucose co-transporter taps into that potential energy by allowing the sodium to move down its concentration gradient into the cell, this converts the potential energy into kinetic energy, which the co-transporter is able to use to do the work of pumping glucose into the cell.
Chemical Energy
On a chemical level, the bonds that hold the atoms of molecules together have potential energy. The type of potential energy that exists within chemical bonds, and is released when those bonds are broken, is called chemical energy. Chemical energy is responsible for providing living cells with energy from food. The release of energy is brought about by breaking the molecular bonds within fuel molecules.
Adenosine Triphosphate
Within the cell, where does energy to power such reactions come from? The answer lies with an energy-supplying molecule called adenosine triphosphate, or ATP. ATP is a small, relatively simple molecule (Figure 7.1.3), but within some of its bonds, it contains the potential for a quick burst of energy that can be harnessed to perform cellular work. This molecule can be thought of as the primary energy currency of cells though it should be noted that ATP is a highly unstable molecule. Unless quickly used to perform work, ATP spontaneously dissociates into ADP + Pi, and the free energy released during this process is lost as heat.
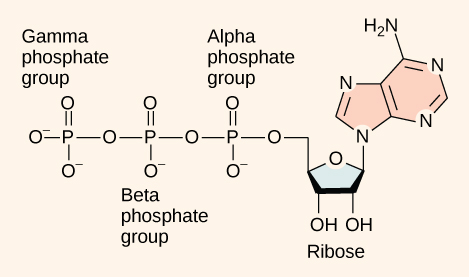
As its name suggests, adenosine triphosphate is comprised of adenosine bound to three phosphate groups (Figure 7.1.3). Adenosine is a nucleoside consisting of the nitrogenous base adenine and a five-carbon sugar, ribose. The three phosphate groups, in order of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma. Together, these chemical groups constitute an energy powerhouse. However, not all bonds within this molecule exist in a particularly high-energy state. Both bonds that link the phosphates are equally high-energy bonds that, when broken, release sufficient energy to power a variety of cellular reactions and processes. These high-energy bonds are the bonds between the second and third (or beta and gamma) phosphate groups and between the first and second phosphate groups. The reason that these bonds are considered “high-energy” is because the products of such bond breaking—adenosine diphosphate (ADP) and one inorganic phosphate group (Pi)—have considerably lower free energy than the reactants: ATP and a water molecule. Because this reaction takes place with the use of a water molecule, it is considered a hydrolysis reaction. In other words, ATP is hydrolyzed into ADP in the following reaction:
\(\text{ATP}+{\text{H}}_{\text{2}}\text{O}\to \text{ADP}+{\text{P}}_{\text{i}}+\text{free energy}\)
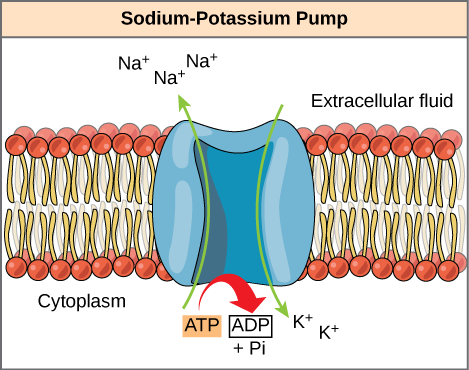
ATP is used to power the majority of energy-requiring cellular reactions. We've already seen it at work when we discussed primary active transport. The sodium-potassium pump, for example, catalyzes the hydrolysis of ATP and captures the released free energy to promote pumping of sodium out of the cell and potassium into the cell. The sodium-potassium pump is an example of energy coupling. The energy derived from exergonic ATP hydrolysis is used to pump sodium and potassium ions across the cell membrane.
Oxidation-Reduction Reactions
Another form of energy coupling that transforms energy in one chemical form into another are oxidation-reduction (redox) reactions, a type of chemical reaction that involves a transfer of electrons between two species. A redox reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting.
Redox reactions are comprised of two parts, a reduced half and an oxidized half, that always occur together. The reduced half gains electrons and the oxidation number decreases, while the oxidized half loses electrons and the oxidation number increases. Simple ways to remember this include the mnemonic devices OIL RIG, meaning "oxidation is loss" and "reduction is gain." There is no net change in the number of electrons in a redox reaction. Those given off in the oxidation half reaction are taken up by another species in the reduction half reaction.
The two species that exchange electrons in a redox reaction are given special names:
- The ion or molecule that accepts electrons is called the oxidizing agent; by accepting electrons, it oxidizes other species.
- The ion or molecule that donates electrons is called the reducing agent; by giving electrons, it reduces the other species.
Hence, what is oxidized is the reducing agent and what is reduced is the oxidizing agent. (Note: The oxidizing and reducing agents can be the same element or compound, as in disproportionation reactions discussed below).
Practice Questions
Glossary
- adenosine triphosphate (ATP)
- primary energy-carrying molecule in cells that stores and supplies energy for cellular processes. Composed of an adenosine backbone and three phosphate groups, alpha beta and gamma.
- oxidation-reduction reaction
- chemical reaction in which one molecule loses electrons (is oxidized) and another gains electrons (is reduced). A common mnemonic used is OIL (oxidation is losing) RIG (reduction is gaining).
sodium potassium pump
membrane protein that uses energy from ATP to actively transport sodium ions out of the cell and potassium ions into the cell, maintaining an electrochemical gradient
Figure Descriptions
Figure 7.1.1. Two panel image. The panel on the left shows a large body of water behind a dam. The panel on the right shows water flowing over rocks. [Return to Figure 7.1.1]
Figure 7.1.2. The image illustrates a Na+/glucose co-transporter within a cellular membrane. The membrane is depicted as a horizontal band composed of two layers with small circular elements, representing the lipid bilayer. A large, blue, cylindrical structure within the membrane represents the co-transporter. Above the membrane, there is a green sphere labeled "Na+" and a red hexagon labeled "Glucose," both positioned to enter the transporter. Two black arrows point downward through the transporter, indicating the movement direction from the outside to the inside of the cell. The words "Outside" and "Inside" are labeled on either side of the membrane. The background is light blue. [Return to Figure 7.1.2]
Figure 7.1.3. The figure depicts ATP (adenosine triphosphate) as a chemical structure. On the right, a pink adenine base (fused rings) is attached to a light-blue five-carbon ribose sugar labeled “Ribose,” with two OH groups shown at the 2′ and 3′ carbons. Extending left from ribose is a linear chain of three phosphate groups; each phosphate is drawn with double-bonded oxygens and negatively charged oxygens. The phosphate nearest the sugar is labeled Alpha phosphate group, the middle is Beta phosphate group, and the terminal phosphate is Gamma phosphate group. The drawing highlights that ATP consists of an adenosine backbone (adenine + ribose) with three attached phosphates. [Return to Figure 7.1.3]
Figure 7.1.4. The figure, titled “Sodium–Potassium Pump,” shows a cross-section of a phospholipid bilayer with red head groups and yellow tails. Above the red head groups on the right is the text "Extracellular fluid." Below the red head groups on the left is the text "Cytoplasm." A large blue membrane protein sits in the center. Two green arrows indicate opposite ion movements: the left arrow points upward/out of the cell to three labels "Na+," and the right arrow points downward/into the cell to two labels "K+." Below the pump, a small label “ATP” with a red curved arrow point to "ADP +Pi" signifies ATP being used. Together the arrows and ATP icon depict the pump coupling ATP hydrolysis to export sodium ions from the cytoplasm and import potassium ions from outside the cell. [Return to Figure 7.1.4]
Licenses and Attributions
"7.1 Forms of Energy" is adapted from "6.2 Potential, Kinetic, Free and Activation Energy" and "6.4 ATP: Adenosine Triphosphate" by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. "7.1 Forms of Energy" is licensed under CC-BY-NC 4.0.
Media Attributions
- Glucose secondary active transport © BioRender adapted by Christelle Sabatier is licensed under a CC BY (Attribution) license
type of energy associated with objects or particles in motion
type of energy that has the potential to do work; stored energy
potential energy in chemical bonds that is released when those bonds are transferred to other molecules
