5.8 Carbon and Nitrogen Cycles
Christelle Sabatier
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe the biogeochemical cycles of carbon and nitrogen.
- Explain how human activities have impacted these cycles and the potential consequences for Earth.
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth’s surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.
The cycling of these elements is interconnected. For example, the movement of water is critical for the leaching of nitrogen and phosphate into rivers, lakes, and oceans. Furthermore, the ocean itself is a major reservoir for carbon. Thus, mineral nutrients are cycled, either rapidly or slowly, through the entire biosphere, from one living organism to another, and between the biotic and abiotic world. In this section we will focus on carbon and nitrogen. Carbon is found in all organic macromolecules and is an important constituent of fossil fuels. Nitrogen is a major component of our nucleic acids and proteins and is critical to human agriculture.
The Nitrogen Cycle
We are surrounded by a lot of nitrogen: N2 comprises approximately 78% of the atmosphere. However, nitrogen in this form is not available to most living organisms. Getting nitrogen into the living world is difficult. Plants and phytoplankton are not equipped to incorporate nitrogen from the atmosphere (which exists as tightly bonded, triple covalent N2). Nitrogen enters the living world via free-living and symbiotic bacteria, which incorporate nitrogen into their macromolecules through nitrogen fixation. Cyanobacteria live in most aquatic ecosystems where sunlight is present; they play a key role in nitrogen fixation. Cyanobacteria are able to use inorganic sources of nitrogen to “fix” nitrogen. Rhizobium bacteria live symbiotically in the root nodules of legumes (such as peas, beans, and peanuts) and provide them with the organic nitrogen they need. Free-living bacteria, such as Azotobacter, are also important nitrogen fixers.
Organic nitrogen is especially important to the study of ecosystem dynamics since many ecosystem processes, such as plant growth and decomposition, are limited by the available supply of nitrogen. As shown in Figure 5.8.1, the nitrogen that enters living systems by nitrogen fixation is successively converted from organic nitrogen back into nitrogen gas by bacteria. This process occurs in three steps in terrestrial systems: ammonification, nitrification, and denitrification. First, the ammonification process converts nitrogenous waste from living animals or from the remains of dead animals into ammonium (NH4+) by certain bacteria and fungi. Second, the ammonium is converted to nitrites (NO2−) by nitrifying bacteria, such as Nitrosomonas, through nitrification. Subsequently, nitrites are converted to nitrates (NO3−) by similar organisms. Third, the process of denitrification occurs, whereby bacteria, such as Pseudomonas and Clostridium, convert the nitrates into nitrogen gas, allowing it to re-enter the atmosphere.
NH4+, NO2–, and NO3– can all be absorbed by the root systems of plants through a process called assimilation. Once in the plant, these forms of nitrogen will be used to produce amino acids and nucleic acids, which in turn contribute to the production of proteins and nucleotides respectively. Limitations of nitrogen in the soil are common and can lead to growth deficits in plants. To complete the cycle, decomposers digesting the proteins and nucleotides present in plants release nitrogen in the form of NH4+, NO2–, and NO3– back into the soil. This is why compost piles often contain lots of nitrogen and can function as a fertilizer that can be used to supplement the soil and promote enhanced plant growth.
Eutrophication
Human activity can release nitrogen into the environment by two primary means: the combustion of fossil fuels, which releases different nitrogen oxides, and by the use of artificial fertilizers in agriculture, which are then washed into lakes, streams, and rivers by surface runoff. Atmospheric nitrogen is associated with several effects on Earth’s ecosystems including the production of acid rain (as nitric acid, HNO3) and greenhouse gas (as nitrous oxide, N2O) potentially causing climate change. A major effect from fertilizer runoff in saltwater and freshwater is eutrophication, a process whereby nutrient runoff causes the excess growth of microorganisms, depleting dissolved oxygen levels and killing ecosystem fauna.
In 1998, the Marine Mammal Center in Sausalito, California, diagnosed the first case of domoic acid toxicity in marine mammals. Since then, periodic algal blooms that lead to high concentrations of the organism Pseudo-nitzschia australis, which produces the toxin domoic acid. These periods of high growth coincide with a combination of events including warmer waters in the Pacific Ocean triggered more and more frequently by climate change along with run off from agricultural fields along the coast that increase the concentration of nitrogen and phosphorus that these organisms need to grow.
Video 5.8.1. Milestones in Science: Domoic Acid by The Marine Mammal Center (California)
Domoic acid is a small molecule that contains nitrogen. When absorbed into the body through feeding, it acts as a neurotoxin targeting neurons that rely on the common neurotransmitter glutamate disrupting the ability of neurons to properly communicate. When taken in low concentrations, this toxin has minimal effects. However, it’s concentration accumulates in the shellfish and small fish such as anchovies and sardines that feed on the toxin producing algae. When predators at higher trophic levels such as sea lions, otters and even humans feed on these contaminated shellfish and fish, they are exposed to higher levels of domoic acid. Exposure to these high levels of toxin can lead to seizure, delirium, and even death. In humans, this is called amnesic shellfish poisoning as the individuals exposed to the toxin display memory deficits.
![Structure diagram of domoic acid. [linked Image Description available]](https://lmu.pressbooks.pub/app/uploads/sites/16/2017/09/Domoic_acid.svg-scaled.png)
Practice Questions
The Carbon Cycle
Carbon is the second most abundant element in living organisms. Carbon is present in all organic molecules, and its role in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain especially high energy, particularly those derived from fossilized organisms, mainly plants, which humans use as fuel. Since the 1800s, the number of countries using massive amounts of fossil fuels has increased. Since the beginning of the Industrial Revolution, global demand for the Earth’s limited fossil fuel supplies has risen; therefore, the amount of carbon dioxide in our atmosphere has increased. This increase in carbon dioxide has been associated with climate change and other disturbances of the Earth’s ecosystems and is a major environmental concern worldwide. Thus, the “carbon footprint” is based on how much carbon dioxide is produced and how much fossil fuel countries consume.
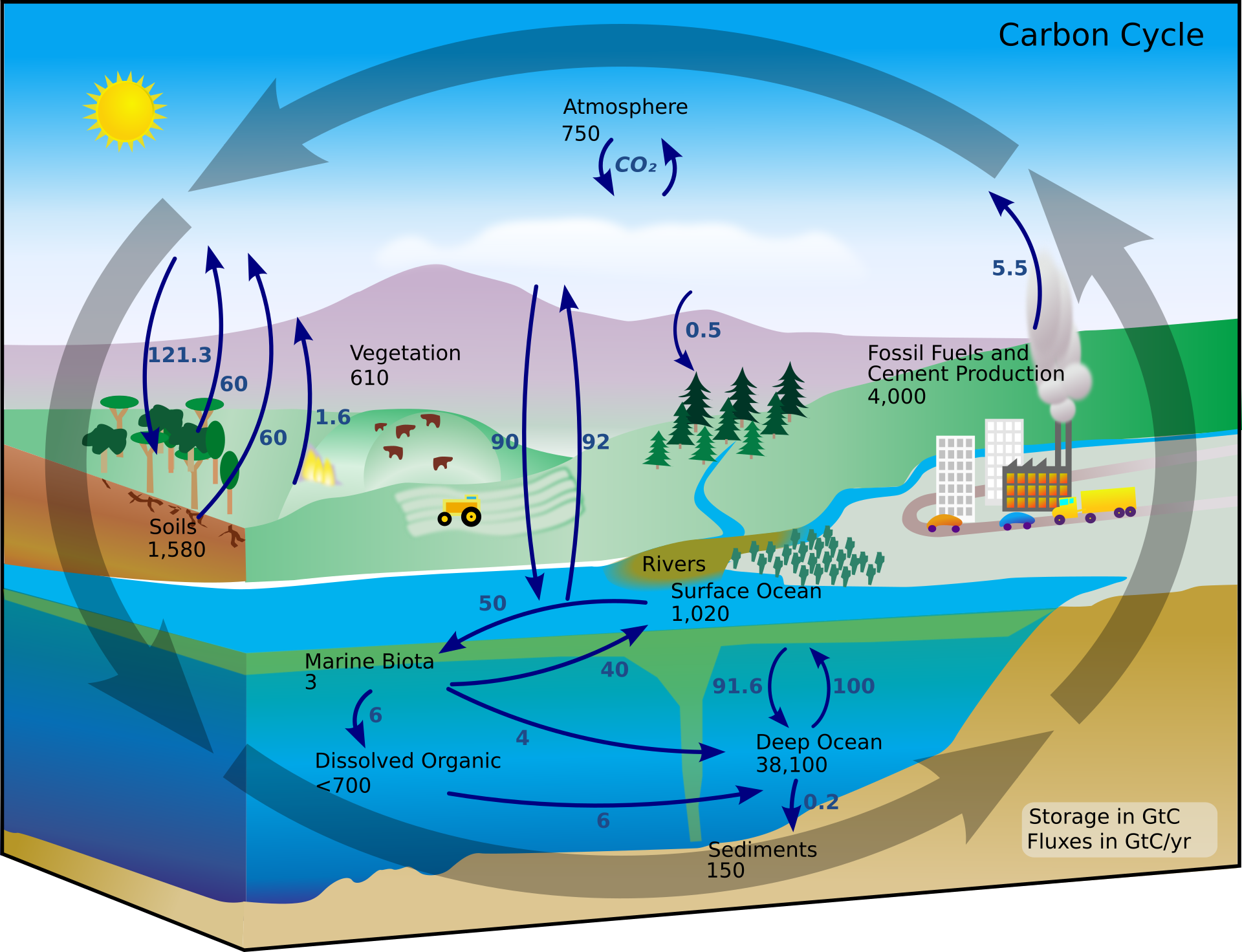
The carbon cycle is most easily studied as two interconnected subcycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes.
The Biological Carbon Cycle
Living organisms are connected in many ways, even between ecosystems. A good example of this connection is the exchange of carbon between autotrophs and heterotrophs within and between ecosystems by way of atmospheric carbon dioxide. Carbon dioxide is the basic building block that most autotrophs use to build multicarbon, high energy compounds, such as glucose. The energy harnessed from the sun is used by these organisms to form the covalent bonds that link carbon atoms together. These chemical bonds thereby store this energy for later use in the process of respiration. Most terrestrial autotrophs obtain their carbon dioxide directly from the atmosphere, while marine autotrophs acquire it in the dissolved form (carbonic acid, H2CO3−). However carbon dioxide is acquired, a by-product of the process is oxygen. The photosynthetic organisms are responsible for depositing approximately 21% oxygen content of the atmosphere that we observe today.
Heterotrophs and autotrophs are partners in biological carbon exchange (especially the primary consumers, largely herbivores). Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them, and breaking them down by respiration to obtain cellular energy, such as ATP. The most efficient type of respiration, aerobic respiration, requires oxygen obtained from the atmosphere or dissolved in water. Thus, there is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Gas exchange through the atmosphere and water is one way that the carbon cycle connects all living organisms on Earth.
The Biogeochemical Carbon Cycle
The movement of carbon through the land, water, and air is complex, and in many cases, it occurs much more slowly geologically than as seen between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs, which include the atmosphere, bodies of liquid water (mostly oceans), ocean sediment, soil, land sediments (including fossil fuels), and the Earth’s interior.
As stated, the atmosphere is a major reservoir of carbon in the form of carbon dioxide and is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each location, and each one affects the other reciprocally. Carbon dioxide (CO2) from the atmosphere dissolves in water and combines with water molecules to form carbonic acid, and then it ionizes to carbonate and bicarbonate ions
On land, carbon is stored in soil as a result of the decomposition of living organisms (by decomposers) or from weathering of terrestrial rock and minerals. This carbon can be leached into the water reservoirs by surface runoff. Deeper underground, on land and at sea, are fossil fuels: the anaerobically decomposed remains of plants that take millions of years to form. Fossil fuels are considered a non-renewable resource because their use far exceeds their rate of formation. A non-renewable resource, such as fossil fuel, is either regenerated very slowly or not at all. Another way for carbon to enter the atmosphere is from land (including land beneath the surface of the ocean) by the eruption of volcanoes and other geothermal systems. Carbon sediments from the ocean floor are taken deep within the Earth by the process of subduction: the movement of one tectonic plate beneath another. Carbon is released as carbon dioxide when a volcano erupts or from volcanic hydrothermal vents.
Carbon dioxide is also added to the atmosphere by the animal husbandry practices of humans. The large numbers of land animals raised to feed the Earth’s growing population results in increased carbon dioxide levels in the atmosphere due to farming practices and the respiration and methane production. This is another example of how human activity indirectly affects biogeochemical cycles in a significant way. Although much of the debate about the future effects of increasing atmospheric carbon on climate change focuses on fossils fuels, scientists take natural processes, such as volcanoes and respiration, into account as they model and predict the future impact of this increase.
Practice Questions
Glossary
denitrification
converts nitrates (NO3–) into nitrogen gas (N2)
nitrification
converts ammonium (NH3) to nitrites (NO2−) and nitrates (NO3–)
eutrophication
a process in which nutrients accumulate in a body of water, resulting in an increased growth of organism
Figure Descriptions
Figure 5.8.1. A colorful schematic shows the nitrogen cycle above and below ground. At the top, right, a cloud with lightning feeds an arrow down to the soil. The arrow points to a box labeled Nitrates (NO₃⁻). Two arrows stretch upward from the box. The first arrow feeds up above ground through soil circles labeled denitrifying bacteria to a blue sky where a box labeled “Atmospheric nitrogen (N₂)” spans the sky. From this box, the arrow moves down the left side of the image back underground to a legume plant with root nodules with a circle filled with red bean-shaped items label “nitrogen-fixing bacteria living in legume root nodules.” The arrow continues deeper underground to another group of circled red bean-shaped items labeled “nitrogen-fixing bacteria. The arrow continues further to a box marked Ammonium (NH₄⁺) and above it is the label “Ammonification.” From there, arrows pass through circles labeled nitrifying bacteria to Nitrites (NO₂⁻) and then to the steps titled Nitrification. The second arrow from the Nitrates box moves up to the ground level and points at a green plant labeled “Plants” and next to it is the label of Assimilation, indicating the carrying of nitrates into plants. The arrow then splits again into two. The first arrow points to a white rabbit sitting on the ground, indicating nitrogen moving through animals, and the other arrow points downward toward a white, central box labeled Decomposers (aerobic and anaerobic bacteria and fungi) points back to ammonium (Ammonification) as organisms die or excrete waste. [Return to Figure 5.8.1]
Figure 5.8.2. The picture is a black-and-white chemistry drawing of domoic acid. It’s shown as a set of lines where each line is a bond and each corner is a carbon atom (hydrogens on carbon are not drawn). At the left is a small ring; attached to it is a longer “tail” with a couple of double bonds (shown as double lines). Sticking out from the backbone are three acid groups written as –COOH and one amine group written as –NH₂ near the ring. Some bonds are drawn as solid wedges or dashed wedges; these just show the 3-D direction of those bonds. Overall, the diagram identifies the parts of the domoic acid molecule, the algal toxin produced by Pseudo-nitzschia, without showing any colors or labels beyond the atom letters. [Return to Figure 5.8.2]
Figure 5.8.3. A landscape-style diagram entitled “Carbon Cycle” summarizes the carbon cycle across land, ocean, and atmosphere. The bottom right has a key that includes the transcribed text “Storage in GtC; Fluzes in GtC/yr.” Broad arrows circle the entire diagram moving counterclockwise, from the sky to the land and sea an back up to the sky again. At the top, a box labeled “Atmosphere (CO₂) 750” sits in the sky with arrows circling counterclockwise around the text “CO₂”; Over the forest at left, a downward arrow labeled 121.3 shows CO₂ taken up by photosynthesis, while two upward arrows labeled 60 and 60 show return to the air from plant and soil respiration; a small flame with 1.6 indicates fire. Land reservoirs are labeled Vegetation 610 and Soils 1,580. To the right, a factory labeled “Fossil Fuels and Cement Production 4,000” releases a plume with 5.5 toward the atmosphere. Above the ocean, exchange arrows show 92 from air to surface waters and 90 back to the air. Ocean boxes read Surface Ocean 1,020 and Deep Ocean 38,100, linked by a large upward arrow 100 and an downward arrow 91.6; smaller internal arrows across the water are labeled 50 from the Surface Ocean to the Marine Biota, and 40 from Marine Biota back to the Surface Ocean. From the Marine Biota, which as a 3 next to it, a downward arrow of 6 points to Dissolved Organic of <700, and another arrow labeled pushes to the right towards Deep Ocean. At the seafloor a box Sediments 150 receives a tiny flux 0.2 from Deep Ocean. Rivers carry carbon from land to the sea. Together the numbered arrows and boxes depict how carbon moves among atmosphere, living things, soils, rocks, and ocean pools, and how human emissions add carbon to the air. [Return to Figure 5.8.3]
Licenses and Attributions
“5.8 Carbon and Nitrogen Cycles” is adapted from “46.3 Biogeochemical Cycles” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “5.8 Carbon and Nitrogen Cycles” is licensed under CC-BY-NC 4.0.
Media Attributions
- Nitrogen_Cycle_2 © Joanjoc adapted by Hattiel is licensed under a CC BY-SA (Attribution ShareAlike) license
- Domoic_acid.svg © Krakatit is licensed under a CC BY (Attribution) license
- Carbon_cycle-cute_diagram.svg © Kevin Saff is licensed under a CC BY (Attribution) license
the process of converting atmospheric nitrogen (N2) into ammonia (NH3) and other nitrogen compounds that plants and other organisms can use.
conversion of ammonium (NH4+) into nitrite (NO2-) and nitrate (NO3-) by soil bacteria
conversion of nitrate (NO3-) into atmospheric nitrogen (N2) by soil bacteria.
a process in which nutrients accumulate in a body of water, resulting in an increased growth of organism
