2.2 Covalent Bonds and Other Molecular Interactions
Learning Objectives
- Differentiate between polar and non-polar covalent bonds using the concept of electronegativity
- Compare and contrast covalent bonds, hydrogen bonds and van der waals interactions
There are two types of covalent bonds: polar and nonpolar. In a polar covalent bond, atoms unequally share the electrons and are attracted more to one nucleus than the other. Because of the unequal electron distribution between the atoms of different elements, a slightly positive (δ+) or slightly negative (δ–) charge develops. This partial charge is an important property of water and accounts for many of its characteristics.
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge. The greater the difference in electronegativity, the more polarized the electron distribution and the larger the partial charges of the atoms.
Figure 2.2.1 shows the electronegativity values of the elements. In general, electronegativity increases from left to right across a period in the periodic table and decreases down a group. Thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities. Metals tend to be less electronegative elements, and the group 1 metals (e.g., sodium) have the lowest electronegativities. Note that noble gases are excluded from this figure because these atoms usually do not share electrons with others atoms since they have a full valence shell.
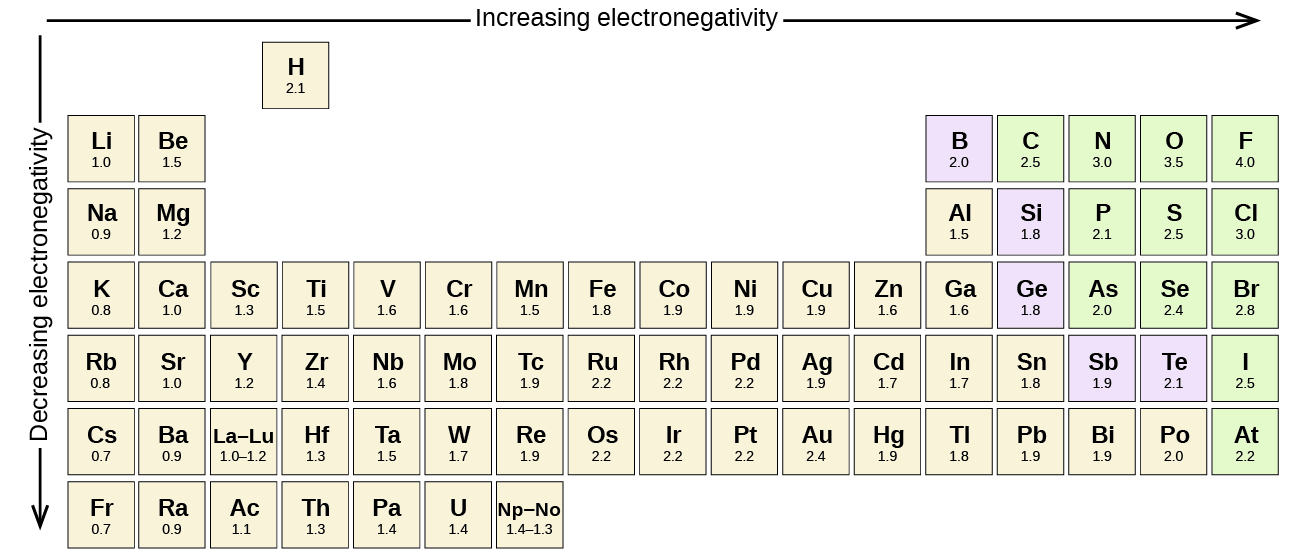
Only a small subset of the atoms are frequently found in biological molecules. Table 2.2.1 highlights their relevant electronegativities.
| Element | Electronegativity |
|---|---|
| Hydrogen (H) | 2.1 |
| Carbon (C) | 2.5 |
| Nitrogen (N) | 3.0 |
| Oxygen (O) | 3.5 |
| Sodium (Na) | 0.9 |
| Phosphorus (P) | 2.1 |
| Sulfur (S) | 2.5 |
| Chloride (Cl) | 3.0 |
Electronegativity and Bond Type
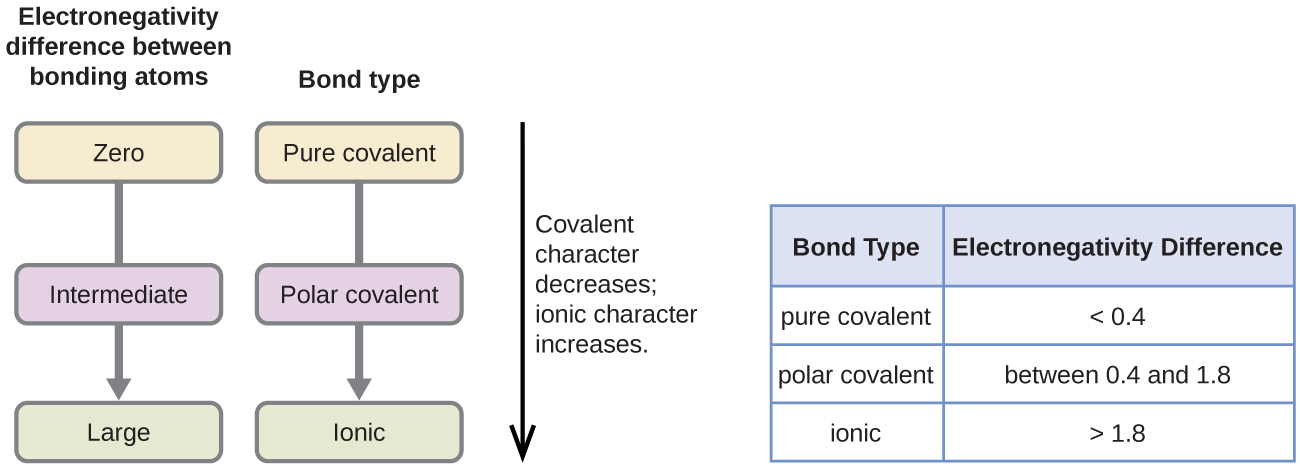
The absolute value of the difference in electronegativity (ΔEN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. When the difference is very small or zero, the bond is covalent and nonpolar. When it is large, the bond is polar covalent or ionic. The absolute values of the electronegativity differences between the atoms in the bonds H–H, H–Cl, and Na–Cl are 0 (nonpolar), 0.9 (polar covalent), and 2.1 (ionic), respectively. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Figure 2.2.2 shows the relationship between electronegativity difference and bond type. This table is just a general guide, however, with many exceptions. For example, the H and F atoms in HF have an electronegativity difference of 1.9, and the N and H atoms in NH3 a difference of 0.9, yet both of these compounds form bonds that are considered polar covalent.
Polar Covalent Bonds
Water is a polar molecule, with the hydrogen atoms acquiring a partial positive charge and the oxygen a partial negative charge. This occurs because the oxygen atom’s nucleus is more attractive to the hydrogen atoms’ electrons than the hydrogen nucleus is to the oxygen’s electrons. Thus, oxygen has a higher electronegativity than hydrogen and the shared electrons spend more time near the oxygen nucleus than the hydrogen atoms’ nucleus, giving the oxygen and hydrogen atoms slightly negative and positive charges, respectively. Another way of stating this is that the probability of finding a shared electron near an oxygen nucleus is more likely than finding it near a hydrogen nucleus. Either way, the atom’s relative electronegativity contributes to developing partial charges whenever one element is significantly more electronegative than the other, and the charges that these polar bonds generate may then be used to form hydrogen bonds based on the attraction of opposite partial charges. (Hydrogen bonds, which we discuss in detail below, are weak bonds between slightly positively charged hydrogen atoms to slightly negatively charged atoms in other molecules.) Since macromolecules often have atoms within them that differ in electronegativity, polar bonds are often present in organic molecules.
Nonpolar Covalent Bonds
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O2) is nonpolar because the electrons distribute equally between the two oxygen atoms.
Methane (CH4) shows another example of a nonpolar covalent bond. Carbon has four electrons in its outermost shell and needs four more to fill it. It obtains these four from four hydrogen atoms, each atom providing one, making a stable outer shell of eight electrons. Carbon and hydrogen do not have the same electronegativity but are similar; thus, nonpolar bonds form. The hydrogen atoms each need one electron for their outermost shell, which is filled when it contains two electrons. These elements share the electrons equally among the carbons and the hydrogen atoms, creating a nonpolar covalent molecule.
Hydrogen Bonds and van der Waals Interactions
Ionic and covalent bonds between elements require energy to break. Ionic bonds are not as strong as covalent, which determines their behavior in biological systems. However, not all bonds are ionic or covalent bonds. Weaker bonds can also form between molecules. Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist. Hydrogen bonds provide many of the critical, life-sustaining properties of water and also stabilize the structures of proteins and DNA, the building block of cells.
When polar covalent bonds containing hydrogen form, the hydrogen in that bond has a slightly positive charge because hydrogen’s electron is pulled more strongly toward the other element and away from the hydrogen. Because the hydrogen is slightly positive, it will be attracted to neighboring negative charges. When this happens, a weak interaction occurs between the hydrogen’s δ+ from one molecule and the molecule’s δ– charge on another molecule with the more electronegative atoms, usually oxygen. Scientists call this interaction a hydrogen bond. This type of bond is common and occurs regularly between water molecules. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. Hydrogen bonds are also responsible for zipping together the DNA double helix.
Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. Van der Waals attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which are not always symmetrical around an atom. For these attractions to happen, the molecules need to be very close to one another. These bonds—along with ionic, covalent, and hydrogen bonds—contribute to the proteins’ three-dimensional structure in our cells that is necessary for their proper function.
Practice Questions
Glossary
electronegativity
a measure of the tendency of an atom to attract electrons (or electron density) towards itself.
polar covalent bonds
covalent bonds between atoms where electrons are not equally shared. Electronegativity difference is generally between 0.4 and 1.8.
non-polar covalent bonds
covalent bonds between atoms where electrons are equally shared. Electronegativity difference is generally below 0.4.
hydrogen bonds
attractive force between partially positive and partially negative atoms that are part of polar covalent bonds in separate molecules.
partial charge
A small electrical charge on part of a molecule that occurs when electrons are shared unequally in a polar covalent bond.
van der waals interactions
weak force between neutral atoms that depends on interaction between their electron clouds.
Figure Descriptions
Figure 2.2.1. The image is a visual representation of the periodic table of elements, illustrating the trend of electronegativity. The elements are arranged in a horizontal and vertical grid.
- General Layout:
- The horizontal axis is labeled “Increasing electronegativity” with an arrow pointing to the right.
- The vertical axis is labeled “Decreasing electronegativity” with an arrow pointing downward.
- Element Representation:
- Each element is displayed in a rectangular box with two pieces of information: the symbol of the element and its electronegativity value.
- Color Coding:
- Boxes with elements in the general representation color (neutral) denote most of the elements.
- Elements in columns 13-16 that are metalloid or non-metals are shaded in light purple and light green.
- Electronegativity Trend:
- The trend shows increasing electronegativity from left to right and decreasing electronegativity from top to bottom. [Return to Figure 2.2.1]
Figure 2.2.2. The image is a diagram explaining the relationship between electronegativity differences between bonding atoms and the resulting types of bonds. On the left, a flowchart shows the electronegativity difference between bonding atoms categorized into three types: Zero, Intermediate, and Large. Each of these categories is associated with a specific bond type.
- Zero electronegativity difference leads to a pure covalent bond.
- Intermediate electronegativity difference leads to a polar covalent bond.
- Large electronegativity difference leads to an ionic bond.
There is a vertical arrow pointing downward next to the flowchart, labeled with the text “Covalent character decreases; ionic character increases,” indicating that as the electronegativity difference increases, the bond character shifts from covalent to ionic.
On the right, there is a table with two columns:
- The left column is labeled “Bond Type” and lists the types of bonds: pure covalent, polar covalent, and ionic.
- The right column is labeled “Electronegativity Difference” and specifies the corresponding ranges for electronegativity differences:
- Pure covalent: < 0.4
- Polar covalent: between 0.4 and 1.8
- Ionic: > 1.8 [Return to Figure 2.2.2]
Licenses and Attributions
“2.2 Covalent Bonds and Other Molecular Interactions” is adapted from “7.2 Covalent Bonding” by Paul Flowers, Klaus Theopold, Richard Langley, and William R. Robinson, PhD for OpenStax Chemistry 2e under CC-BY 4.0. “2.2 Covalent Bonds and Other Molecular Interactions” is licensed under CC-BY-NC 4.0.
Media Attributions
- Electronegativity table © OpenStax Chemistry 2e is licensed under a CC BY (Attribution) license
- CNX_Chem_01_02_MattType © OpenStax Chemistry 2e is licensed under a CC BY (Attribution) license
a measure of the tendency of an atom to attract electrons (or electron density) towards itself.
attractive force between partially positive and partially negative atoms that are part of polar covalent bonds in separate molecules.
weak force between neutral atoms that depends on interaction between their electron clouds.
