2.3 Water
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe the properties of water that are critical to maintaining life.
- Explain why water is an excellent solvent.
- Predict the water solubility of small molecules based on their structures.
Why do scientists spend time looking for water on other planets? Why is water so important? It is because water is essential to life as we know it. Water is one of the more abundant molecules and the one most critical to life on Earth. Water comprises approximately 60% to 70% of the human body. Without it, life as we know it simply would not exist.
The polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the processes of life. Life originally evolved in a watery environment, and most of an organism’s cellular chemistry and metabolism occur inside the watery contents of the cell’s cytoplasm. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions that leads to generating pH. Understanding these characteristics of water helps to elucidate its importance in maintaining life.
Water Polarity
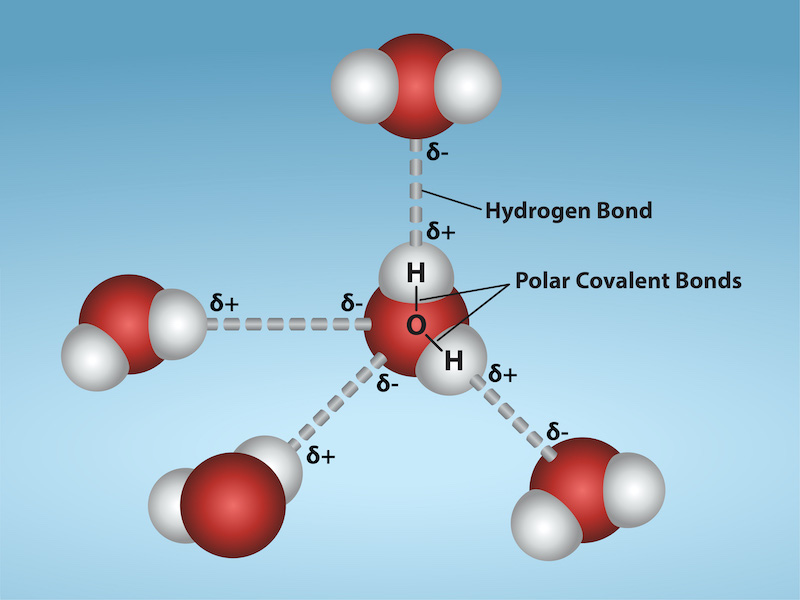
One of water’s important properties is that it is composed of polar molecules: the hydrogen and oxygen within water molecules (H2O) form polar covalent bonds. While there is no net charge to a water molecule, water’s polarity creates a slightly positive charge on hydrogen and a slightly negative charge on oxygen, contributing to water’s properties of attraction. Water generates charges because oxygen is more electronegative than hydrogen, making it more likely that a shared electron would be near the oxygen nucleus than the hydrogen nucleus, thus generating the partial negative charge near the oxygen.
As a result of water’s polarity, each water molecule attracts other water molecules because of the opposite charges between water molecules, forming hydrogen bonds. Water also attracts or is attracted to other polar molecules and ions. We call a polar substance that interacts readily with or dissolves in water hydrophilic (hydro- = “water”; -philic = “loving”). In contrast, nonpolar molecules such as oils and fats do not interact well with water, as Figure 2.3.1 shows. A good example of this is vinegar and oil salad dressing (an acidic water solution). We call such nonpolar compounds hydrophobic (hydro- = “water”; -phobic = “fearing”).
Water’s States: Gas, Liquid, and Solid
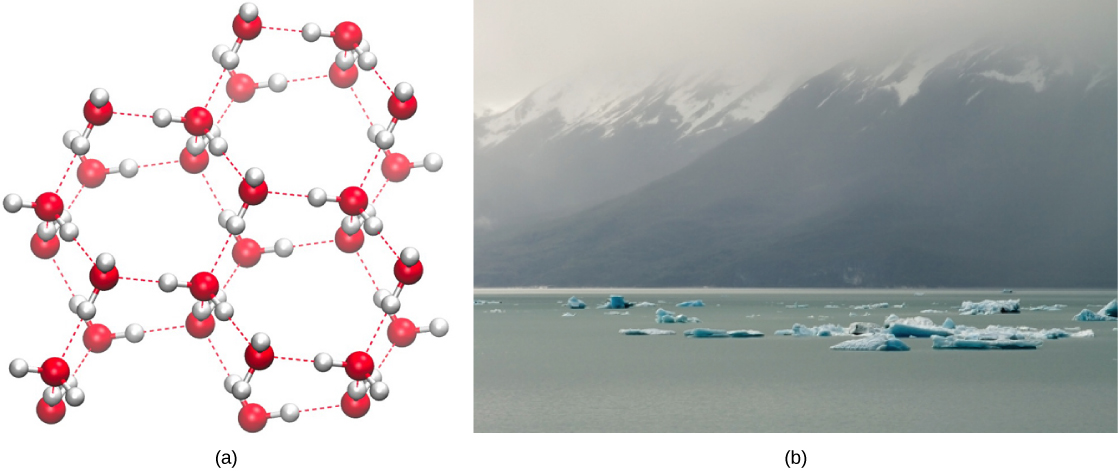
The formation of hydrogen bonds is an important quality of the liquid water that is crucial to life as we know it. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high water content, understanding these chemical features is key to understanding life. In liquid water, hydrogen bonds constantly form and break as the water molecules slide past each other. The water molecules’ motion (kinetic energy) causes the bonds to break due to the heat contained in the system. When the heat rises as water boils, the water molecules’ higher kinetic energy causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas (steam or water vapor). Alternatively, when water temperature reduces and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding (there is not enough energy to break the hydrogen bonds) that makes ice less dense than liquid water, a phenomenon that we do not see when other liquids solidify.
Water’s lower density in its solid form is due to the way hydrogen bonds orient as they freeze: the water molecules push farther apart compared to liquid water. With most other liquids, solidification when the temperature drops includes lowering kinetic energy between molecules, allowing them to pack even more tightly than in liquid form and giving the solid a greater density than the liquid.
The lower density of ice, as Figure 2.3.2 depicts, an anomaly causes it to float at the surface of liquid water, such as in an iceberg or ice cubes in a glass of water. In lakes and ponds, ice will form on the water’s surface creating an insulating barrier that protects the animals and plant life in the pond from freezing. Without this insulating ice layer, plants and animals living in the pond would freeze in the solid block of ice and could not survive. The expansion of ice relative to liquid water causes the detrimental effect of freezing on living organisms. The ice crystals that form upon freezing rupture the delicate membranes essential for living cells to function, irreversibly damaging them. Cells can only survive freezing if another liquid like glycerol temporarily replaces the water in them.
Water’s Solvent Properties
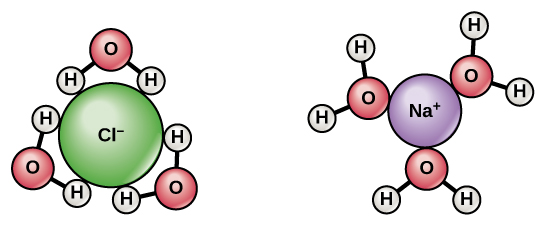
Since water is a polar molecule with slightly positive and slightly negative charges, ions and polar molecules can readily dissolve in it. Therefore, we refer to water as a solvent, a substance capable of dissolving other polar molecules and ionic compounds. The charges associated with these molecules will form hydrogen bonds with water, surrounding the particle with water molecules. We refer to this as a sphere of hydration, or a hydration shell, as Figure 2.3.3 illustrates and serves to keep the particles separated or dispersed in the water.
When we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Dissociation occurs when atoms or groups of atoms break off from molecules and form ions. Consider table salt (NaCl, or sodium chloride): when we add NaCl crystals to water, the NaCl molecules dissociate into Na+ and Cl– ions, and spheres of hydration form around the ions, as Figure 2.3.3 illustrates. The partially negative charge of the water molecule’s oxygen surrounds the positively charged sodium ion. The hydrogen’s partially positive charge on the water molecule surrounds the negatively charged chloride ion.
Determining the solubility of molecules in water
We can tell a lot about the water solubility of a molecule from its molecular structure. We can use the electronegativity rules described in chapter 2.2 to identify polar and non-polar covalent bonds in a molecule. If the polar bonds are spread out over the whole molecule, it is soluble in water and considered hydrophilic. If the majority of the bonds are nonpolar, the molecule is insoluble in water and is considered hydrophobic. If one part of the molecule is hydrophilic and another separate part is hydrophobic, the molecule is considered amphipathic. These molecules have a low solubility in water. See the video below for an example on how
to do this:
Video 2.3.1 Determining the water solubility of a molecule by Christelle Sabatier
Practice Questions
Glossary
solubility
ability of a substance, the solute, to form a solution with another substance, the solvent.
hydrophobic
molecule that does not have the ability to bond with water; “water-hating”
hydrophilic
molecule with the ability to interact with water; “water-loving”
amphipathic
having both hydrophilic and hydrophobic parts.
Figure Descriptions
Figure 2.3.1. The image depicts a molecular structure diagram of water molecules, represented with a light blue background. At the center, there is a water molecule composed of one large red sphere, symbolizing oxygen, and two smaller white spheres, representing hydrogen atoms. These atoms are connected by solid lines labeled “Polar Covalent Bonds,” indicating the type of bonds between the atoms. Surrounding the central water molecule, three additional water molecules are shown. Each adjacent molecule consists of similar red and white spheres connected, with dashed lines labeled “Hydrogen Bond” connecting them to the central molecule. The diagram includes delta symbols (δ) near each atom, indicating partial positive (δ+) and partial negative (δ-) charges. The hydrogen atoms are labeled with δ+ signs, while the oxygen atoms are labeled with δ- signs. [Return to Figure 2.3.1]
Figure 2.3.2. The image consists of two sections. On the left is a molecular diagram labeled “(a)” which depicts a model of a water molecule structure. The structure consists of spheres connected by rods, representing atoms and their bonds. The spheres vary in color, with red spheres associated with oxygen and white spheres for hydrogen, connected by gray rods indicating covalent bonds, and red dashed lines representing hydrogen bonds. On the right is a labeled photograph “(b)” showing a serene landscape of an icy body of water with several small icebergs scattered across its surface. In the background, a range of dark mountains is partially obscured by low-hanging clouds, under a sky that is overcast and gray. The water is a muted greenish-blue, reflecting the icy tones of the scattered icebergs. [Return to Figure 2.3.2]
Figure 2.3.3. The image displays two molecular structures. On the left is a chloride ion (Cl⁻), represented by a large green sphere with the label “Cl⁻”. Surrounding it are three water molecules, each depicted as a red “O” sphere bonded to two smaller white “H” spheres, indicating hydrogen atoms. The water molecules form a triangular shape around the Cl⁻ ion. On the right is a sodium ion (Na⁺), shown as a smaller purple sphere labeled “Na⁺”. It is surrounded by three water molecules similar to those around the chloride ion. These water molecules are arranged with their oxygen atoms facing inward toward the sodium ion, forming a similar triangular shape. [Return to Figure 2.3.3]
Licenses and Attributions
“2.3 Water” is adapted from “2.2 Water” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “2.3 Water” is licensed under CC-BY-NC 4.0.
Media Attributions
- 2.15 Water Molecule – Hydrogen Bond-XY © Rao, A., Fletcher, S., Ryan, K., Tag, A. and Hawkins, A. Department of Biology, Texas A&M University is licensed under a CC BY (Attribution) license
- 1A.B.3 Solid Water © credit a: modification of work by Jane Whitney, image created using Visual Molecular Dynamics (VMD) software Humphrey W., A. Dalke, and K. Schulten, “VMD—Visual Molecular Dynamics,” Journal of Molecular Graphics 14 (1996): 33-38.; credit b: modification of work by Carlos Ponte is licensed under a CC BY (Attribution) license
- 1A.B.3 Solutes in water © Clark, Douglas and Choi is licensed under a CC BY (Attribution) license
the ability of a substance, the solute, to form a solution with another substance, the solvent.
molecule with the ability to interact with water; “water-loving”
molecule that does not have the ability to bond with water; “water-hating”
having both hydrophilic and hydrophobic parts.
