12.2 Energy Flow Through Ecosystems
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe how organisms acquire energy in a food web and in associated food chains.
- Explain how the efficiency of energy transfers between trophic levels affects ecosystem structure and dynamics.
- Discuss trophic levels and how ecological pyramids are used to model them.
All living things require energy in one form or another. Energy is required by most complex metabolic pathways (often provided through reactions with adenosine triphosphate [pb_glossary id="5142"]ATP[/pb_glossary]), especially those responsible for building large molecules from smaller compounds, and life itself is an energy-driven process. Living organisms would not be able to assemble macromolecules (proteins, lipids, nucleic acids, and complex carbohydrates) from their monomeric subunits without a constant energy input.
It is important to understand how organisms acquire energy and how that energy is passed from one organism to another through food webs and their constituent food chains. Food webs illustrate how energy flows directionally through ecosystems, including how efficiently organisms acquire it, use it, and how much remains for use by other organisms of the food web.
How Organisms Acquire Energy in a Food Web
Energy is acquired by living things in three ways: photosynthesis, chemosynthesis, and the consumption and digestion of other living or previously living organisms by heterotrophs (Figure 12.2.1).
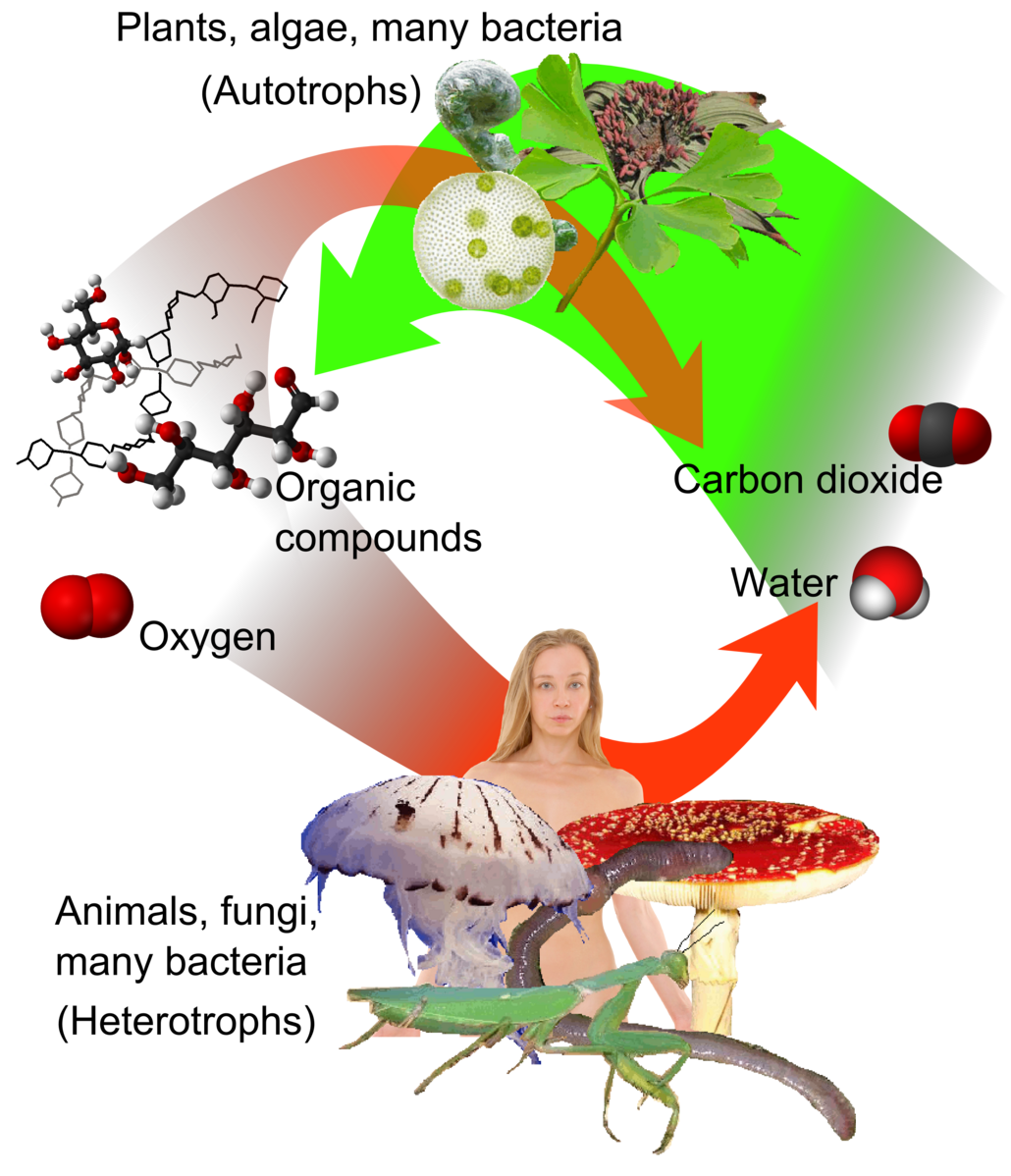
Photosynthetic and chemosynthetic organisms are both grouped into a category known as autotrophs: organisms capable of synthesizing their own food (more specifically, capable of using inorganic carbon as a carbon source). Photosynthetic autotrophs (photoautotrophs) use sunlight as an energy source, whereas chemosynthetic autotrophs (chemoautotrophs) use inorganic molecules as an energy source. Autotrophs are critical for all ecosystems. Without these organisms, energy would not be available to other living organisms and life itself would not be possible.
Photoautotrophs, such as plants, algae, and photosynthetic bacteria, serve as the energy source for a majority of the world’s ecosystems. Photoautotrophs harness the solar energy of the sun by converting it to chemical energy in the form of ATP (and NADP). The energy stored in ATP is used to synthesize complex organic molecules, such as glucose.
Chemoautotrophs are primarily bacteria that are found in rare ecosystems where sunlight is not available, such as in those associated with dark caves or hydrothermal vents at the bottom of the ocean. Many chemoautotrophs in hydrothermal vents use hydrogen sulfide (H2S), which is released from the vents as a source of chemical energy. This allows chemoautotrophs to synthesize complex organic molecules, such as glucose, for their own energy and in turn supplies energy to the rest of the ecosystem.
Productivity Within Trophic Levels
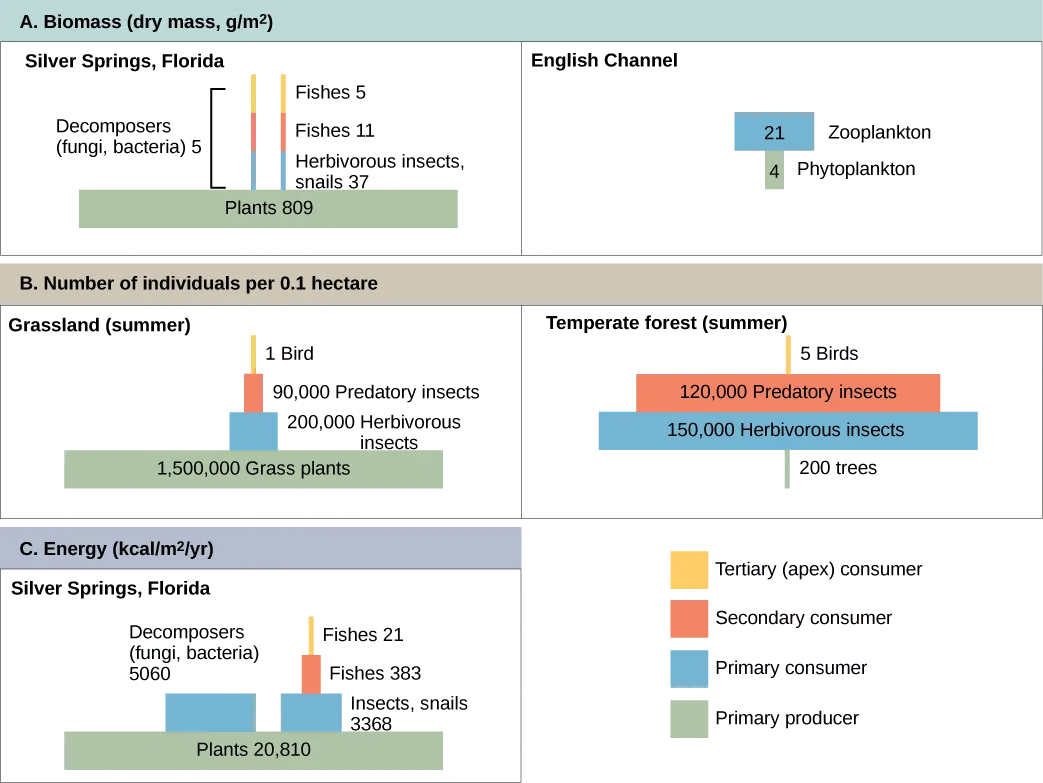
Productivity within an ecosystem can be defined as the rate of generation of biomass, usually expressed in units of mass per volume (unit surface) per unit of time. Biomass is the total mass, in a unit area at the time of measurement, of living or previously living organisms within a trophic level. Ecosystems have characteristic amounts of biomass at each trophic level. For example, in the English Channel ecosystem the primary producers account for a biomass of 4 g/m2 (grams per square meter), while the primary consumers exhibit a biomass of 21 g/m2.
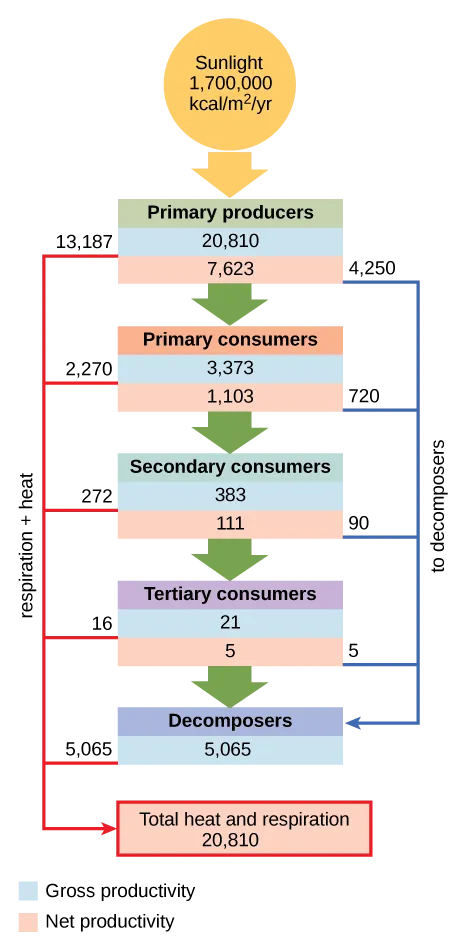
The productivity of the primary producers is especially important in any ecosystem because these organisms bring energy to other living organisms by photoautotrophy or chemoautotrophy. The rate at which photosynthetic primary producers incorporate energy from the sun is called gross primary productivity. An example of gross primary productivity is shown in the compartment diagram of energy flow within the Silver Springs aquatic ecosystem as shown (Figure 12.7). In this ecosystem, the total energy accumulated by the primary producers (gross primary productivity) was shown to be 20,810 kcal/m2/yr.
Because all organisms need to use some of this energy for their own functions (like respiration and resulting metabolic heat loss) scientists often refer to the net primary productivity of an ecosystem. Net primary productivity is the energy that remains in the primary producers after accounting for the organisms’ respiration and heat loss. The net productivity is then available to the primary consumers at the next trophic level. In our Silver Springs example, 13,187 of the 20,810 kcal/m2/yr were used for respiration or were lost as heat by the primary producers, leaving 7,633 kcal/m2/yr of energy for use by the primary consumers.
Ecological Efficiency: The Transfer of Energy Between Trophic Levels
As illustrated in (Figure 12.2.2), as energy flows from primary producers through the various trophic levels, the ecosystem loses large amounts of energy. The main reason for this loss is the second law of thermodynamics, which states that whenever energy is converted from one form to another, there is a tendency toward disorder (entropy) in the system. In biologic systems, this energy takes the form of metabolic heat, which is not passed on between trophic levels when the organisms consume other organisms but rather lost from the system. In the Silver Springs ecosystem example (Figure 12.2.2), we see that the primary consumers produced 1103 kcal/m2/yr from the 7618 kcal/m2/yr of energy available to them from the primary producers. The measurement of energy transfer efficiency between two successive trophic levels is termed the trophic level transfer efficiency (TLTE) and is defined by the formula:
In Silver Springs, the TLTE between the first two trophic levels was approximately 14.48%. The low efficiency of energy transfer between trophic levels is usually the major factor that limits the length of food chains observed in a food web. The fact is, after four to six energy transfers, there is not enough energy left to support another trophic level.
Ecologists have many different methods of measuring energy transfers within ecosystems. Measurement difficulty depends on the complexity of the ecosystem and how much access scientists have to observe the ecosystem. In other words, some ecosystems are more difficult to study than others, and sometimes the quantification of energy transfers has to be estimated.
Other parameters are important in characterizing energy flow within an ecosystem. Net production efficiency (NPE) allows ecologists to quantify how efficiently organisms of a particular trophic level incorporate the energy they receive into biomass. NPE describes the proportion of ingested food that is incorporated into new biomass by the consumer. It is calculated using the following formula:
Net consumer productivity is the rate at which consumers in an ecosystem convert the energy they obtain from food into their own biomass.. Assimilation is the biomass of the organisms consumed from one trophic level that is incorporated into another trophic level after accounting for the energy lost due to incomplete ingestion of food, energy used for respiration, and energy lost as waste. Incomplete ingestion refers to the fact that some consumers eat only a part of their food. For example, when a lion kills an antelope, it will eat everything except the hide and bones. The lion is missing the energy-rich bone marrow inside the bone, so the lion does not make use of all the calories its prey could provide.
Thus, NPE measures how efficiently each trophic level uses and incorporates the energy from its food into biomass to fuel the next trophic level. In general, cold-blooded animals (ectotherms), such as invertebrates, fish, amphibians, and reptiles, use less of the energy they obtain for respiration and heat than warm-blooded animals (endotherms), such as birds and mammals. The extra heat generated in endotherms, although an advantage in terms of the activity of these organisms in colder environments, is a major disadvantage in terms of NPE. Therefore, many endotherms have to eat more often than ectotherms to get the energy they need for survival. In general, NPE for ectotherms is an order of magnitude (10x) higher than for endotherms. For example, the NPE for a caterpillar eating leaves has been measured at 18%, whereas the NPE for a squirrel eating acorns may be as low as 1.6%.
The inefficiency of energy use by warm-blooded animals has broad implications for the world's food supply. It is widely accepted that the meat industry uses large amounts of crops to feed livestock, and because the NPE is low, much of the energy from animal feed is lost. For example, it costs about $0.01 to produce 1,000 dietary calories (kcal) of corn or soybeans, but approximately $0.19 to produce a similar number of calories growing cattle for beef consumption. The same energy content of milk from cattle is also costly, at approximately $0.16 per 1,000 kcal. Much of this difference is due to the low NPE of cattle. Thus, there has been a growing movement worldwide to promote the consumption of nonmeat and nondairy foods so that less energy is wasted feeding animals for the meat industry.
Modeling Ecosystems Energy Flow: Ecological Pyramids
The structure of ecosystems can be visualized with ecological pyramids, which were first described by the pioneering studies of Charles Elton in the 1920s. Ecological pyramids show the relative amounts of various parameters (such as number of organisms, energy, and biomass) across trophic levels.
Pyramids of numbers can be either upright or inverted, depending on the ecosystem. As shown in Figure 12.2.3, typical grassland during the summer has a base of many plants, and the numbers of organisms decrease at each trophic level. However, during the summer in a temperate forest, the base of the pyramid consists of few trees compared with the number of primary consumers, mostly insects. Because trees are large, they have great photosynthetic capability, and dominate other plants in this ecosystem to obtain sunlight. Even in smaller numbers, primary producers in forests are still capable of supporting other trophic levels.
Another way to visualize ecosystem structure is with pyramids of biomass. This pyramid measures the amount of energy converted into living tissue at the different trophic levels. Using the Silver Springs ecosystem example, this data exhibits an upright biomass pyramid (Figure 12.2.3), whereas the pyramid from the English Channel example is inverted. The plants (primary producers) of the Silver Springs ecosystem make up a large percentage of the biomass found there. However, the phytoplankton in the English Channel example make up less biomass than the primary consumers, the zooplankton. As with inverted pyramids of numbers, this inverted pyramid is not due to a lack of productivity from the primary producers, but results from the high turnover rate of the phytoplankton. The phytoplankton are consumed rapidly by the primary consumers, thus, minimizing their biomass at any particular point in time. However, phytoplankton reproduce quickly, thus they are able to support the rest of the ecosystem.
Pyramid ecosystem modeling can also be used to show energy flow through the trophic levels. Notice that these numbers are the same as those used in the energy flow compartment diagram in (Figure 12.2.2). Pyramids of energy are always upright, and an ecosystem without sufficient primary productivity cannot be supported. All types of ecological pyramids are useful for characterizing ecosystem structure. However, in the study of energy flow through the ecosystem, pyramids of energy are the most consistent and representative models of ecosystem structure (Figure 12.2.3).
Consequences of Food Webs: Biological Magnification
One of the most important environmental consequences of ecosystem dynamics is biomagnification. Biomagnification is the increasing concentration of persistent, toxic substances in organisms at each trophic level, from the primary producers to the apex consumers. This occurs because of two factors: (1) Because ecological efficiencies are low, each trophic level has to consume a large amount of biomass from the trophic levels below to make a small amount of biomass and (2) once consumed, certain substances are difficult or impossible to
remove from a body, this is particularly true for hydrophobic molecules.
Many substances have been shown to bioaccumulate, including the pesticide dichlorodiphenyltrichloroethane (DDT), which was described in the 1960s bestseller, Silent Spring, by marine biologist Rachel Carson. DDT was a commonly used pesticide before its dangers became known. It is a hydrophobic molecule and tends to accumulate in the fat tissues of organisms that are exposed to it. In some aquatic ecosystems, organisms from each trophic level consumed many organisms of the lower level, which caused DDT to increase in birds (apex consumers) that ate fish. One 1967 study found that phytoplankton in an estuary on the East Coast of the United States had a DDT concentration of 0.04 ppb. Since zooplankton has to consume 10 mg of phytoplankton to grow to its adult size of 1 mg, it will end up with an estimated DDT concentration of 0.4 ppb. The small fish that feed on that zooplankton, will accumulate 4 ppb of DDT for every 1 mg of biomass and so on. The study found that in carnivorous birds, DDT concentrations had 10 - 100 times more DDT than the fish (40 - 400 ppb) (Woodwell et al, 1967). Such high concentrations of DDT lead to significant problems for the birds, including highly fragile eggshells. This effect increased egg breakage during nesting and was shown to have adverse effects on these bird populations. Carson's combination of scientific knowledge and illuminating writing helped raise awareness about overall environmental issues as well as the specifics of the pesticide. The use of DDT was banned in the United States in the 1970s.
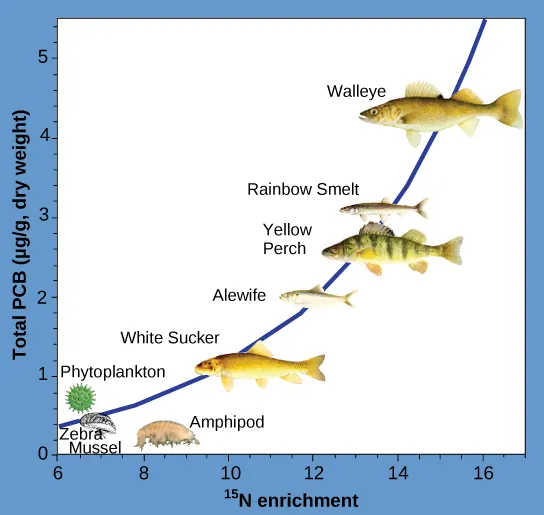
Other substances that biomagnify are polychlorinated biphenyls (PCBs), which were used in coolant liquids in the United States until their use was banned in 1979, and heavy metals, such as mercury, lead, and cadmium. These substances were best studied in aquatic ecosystems, where fish species at different trophic levels accumulate toxic substances brought through the ecosystem by the primary producers. As illustrated in a study performed by the National Oceanic and Atmospheric Administration (NOAA) in the Saginaw Bay of Lake Huron (Figure 12.2.4), PCB concentrations increased from the ecosystem’s primary producers (phytoplankton) through the different trophic levels of fish species. The apex consumer (walleye) has more than four times the amount of PCBs compared to phytoplankton. Also, based on results from other studies, birds that eat these fish may have PCB levels at least one order of magnitude higher than those found in the lake fish.
Other concerns have been raised by the accumulation of heavy metals, such as mercury and cadmium, in certain types of seafood. The United States Environmental Protection Agency (EPA) recommends that pregnant people and young children should not consume any swordfish, shark, king mackerel, or tilefish because of their high mercury content. These individuals are advised to eat fish low in mercury: salmon, tilapia, shrimp, pollock, and catfish. Biomagnification is a good example of how ecosystem dynamics can affect our everyday lives, even influencing the food we eat.
Practice Questions
Glossary
autotrophs
organisms that produce their own food
heterotrophs
organisms that consume other organisms for food
ecological efficiency
proportion of energy transferred between trophic levels in an ecosystem
Reference
Howard T. Odum. (1957). Trophic structure and productivity of Silver Springs, Florida. Ecological Monographs, 27(1):47–112.
Woodwell, G. M., C. F. Wurster Jr, and P. A. Isaacson. 1967. “DDT Residues in an East Coast Estuary: A Case of Biological Concentration of a Persistent Insecticide.” Science (New York, N.Y.) 156 (3776): 821–24.
Figure Descriptions
Figure 12.2.1. The image depicts a conceptual representation of a biological cycle involving humans, plants, and fungi (heterotrophs) and plants, algae, and bacteria (autotrophs). Surrounding the figure, various organisms and chemical structures are interconnected by a pair of large, curved arrows—one green and one orange—forming a cycle. The top left features molecular models and structural diagrams, indicating chemical compounds. The autotrophs absorb and transform inorganic compounds such as carbon dioxide and produce organic molecules and oxygen. The heterotrophs through feeding or decomposition access these organic molecules and use oxygen for their own cellular respiration (the orange arrow). Both autotrophs and heterotrophs respire and produce CO2 thus completing the cycle. [Return to Figure 12.2.1]
Figure 12.2.2. This conceptual model illustrates energy flow through the Silver Springs, Florida, ecosystem as studied by Howard T. Odum. A large yellow arrow at the top represents incoming sunlight, labeled 1,700,000 kcal/m²/yr. Below that, primary producers receive 20,810 kcal/m²/yr of energy, and 7,623 kcal/m²/yr flows to decomposers. Next, primary consumers receive 3,373 kcal/m²/yr, with 1,103 kcal/m²/yr going to decomposers. Secondary consumers receive 383 kcal/m²/yr, transferring 111 kcal/m²/yr to decomposers. Tertiary consumers receive 21 kcal/m²/yr, and pass 5 kcal/m²/yr to decomposers. Finally, decomposers are shown to process 5,065 kcal/m²/yr in total. Each level is connected by green arrows pointing downward, representing energy transfer to the next trophic level. Red arrows show energy lost as total heat and respiration, amounting to 20,810 kcal/m²/yr at the bottom of the diagram. Blue arrows represent energy flowing to decomposers from each level. The model demonstrates how energy decreases at each successive trophic level, with much of the energy lost as heat or passed to decomposers. It provides a holistic view of energy input, utilization, and dissipation in an ecosystem. [Return to Figure 12.2.2]
Figure 12.2.3. Section A, biomass, indicated by dry mass g/m2. On the left is a pyramid diagram of dry biomass in grams per meter squared in Silver Springs, Florida. The biomass of plants is 809. The biomass of primary consumers, including herbivorous insects and snails is 37. The biomass of secondary consumer fishes is 11, and the biomass of tertiary consumer fishes is 5. Primary, secondary and tertiary decomposers have a combined biomass of 5. On the right is a pyramid diagram of dry biomass in grams per meter squared in the English Channel. The biomass is 4 phytoplankton and 21 zooplankton. Section B, number of individuals per 0.1 hectare. On the left is a pyramid diagram of the number of individuals per 0.1 hectare in a summer grassland. There are 1,500,000 grass plants, 200,000 herbivorous insects, 90,000 predatory insects, and 1 bird. On the right is a pyramid diagram of organisms per 0.1 hectare in a temperate forest. There are 200 trees, 150,000 herbivorous insects, 120,000 predatory insects, and 5 birds. Section C, energy in kcal/m2/year. In Silver Springs Florida, the energy of plants is 20,810. The energy of primary consumers, including insects and snails, is 3,368. The energy of secondary consumer fishes is 383, and the energy of tertiary consumer fishes is 21. The energy of decomposers, including fungi and bacteria, is 5,060. [Return to Figure 12.2.3]
Figure 12.2.4. This line graph shows the relationship between PCB concentration (y-axis, measured in micrograms per gram dry weight) and ¹⁵N enrichment (x-axis, a proxy for trophic level) in organisms from the Saginaw Bay ecosystem of Lake Huron. The x-axis ranges from 6 to 16 and the y-axis ranges from 0 to 5.5. A curved blue line represents the increasing accumulation of PCBs as organisms occupy higher trophic levels. At the lower left, phytoplankton, zebra mussels, and amphipods have the lowest PCB concentrations (near 0 to 1 µg/g) and the lowest ¹⁵N enrichment values. Slightly higher on the curve are white sucker and alewife, with moderate PCB levels around 1 to 2 µg/g. Further along the curve, yellow perch and rainbow smelt show higher concentrations, ranging from 2.5 to 3.5 µg/g. At the highest trophic level, walleye exhibit the greatest PCB accumulation, with concentrations above 5 µg/g and the highest nitrogen enrichment. The chart illustrates biomagnification, where PCBs become more concentrated as they move up the food chain. Organisms higher on the trophic ladder, particularly predatory fish, carry a higher toxic burden than those at the base of the food web. [Return to Figure 12.2.4]
Licenses and Attributions
"12.2 Energy Flow through Ecosystems" is adapted from "46.2 Energy Flow through Ecosystems" by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. "12.2 Energy Flow through Ecosystems" is licensed under CC-BY-NC 4.0.
Media Attributions
- 1A.D.12 Auto-and_heterotrophs © Derivative by Mikael Häggström, using originals by Laghi l, BorgQueen, Benjah-bmm27, Rkitko, Bobisbob, Jacek FH, Laghi L and Jynto is licensed under a CC BY-SA (Attribution ShareAlike) license
- 1A.D.12 Silver Springs model © OpenStax is licensed under a CC BY-SA (Attribution ShareAlike) license
- Ecological Pyramids © OpenStax is licensed under a CC BY-SA (Attribution ShareAlike) license
- 1A.D.12 PCB concentrations in Lake Huron © Patricia Van Hoof, NOAA, GLERL adapted by OpenStax is licensed under a CC BY-SA (Attribution ShareAlike) license
high energy molecule used as currency for cellular processes
organism that consumes organic substances or other organisms for food
organisms capable of synthesizing their own food
Measure of randomness
Measure of energy transferred between two trophic levels
