O1. Malaria as a Systems Biology Challenge
Craig Stephens
Learning Objectives
By the end of this chapter, you will be able to do the following:
- Motivate the need for a systems approach to develop solutions for malaria
- Describe the Plasmodium parasite lifecycle in humans and mosquitoes
- Identify the stages of the parasite lifecycle that correspond with clinical symptoms in humans
- Describe antimalarial drugs and their mechanisms of action in Plasmodium
- Describe interventions at the populations level that can curb the spread of malaria
Malaria is one of the world’s most significant infectious diseases. Caused by single-celled protozoan parasites of the genus Plasmodium, it infects hundreds of millions of people annually and leads to hundreds of thousands of deaths, especially among young children in sub-Saharan Africa. Malaria is a vector-borne disease, meaning that the disease-causing Plasmodium parasite is actually delivered into humans by the bite of an infected insect (the vector), in this case a mosquito. Humans largely cannot transmit malaria directly to each other. Despite decades of control efforts, malaria persists due to its biological complexity and its intimate relationship with both humans and mosquitoes. Understanding malaria requires more than just studying individual cells; it demands a systems-level approach, integrating interactions among the parasite, its human host, and the mosquito vector.
1. Epidemiology of Malaria
Malaria remains one of the most significant global health challenges, particularly in tropical and subtropical regions. In 2023, the World Health Organization (WHO) estimated 263 million malaria cases and 597,000 deaths worldwide, with the majority occurring in sub-Saharan Africa. Malaria is endemic in 83 countries, with the WHO African Region bearing the brunt of the disease burden. In 2023, this region accounted for approximately 94% of all malaria cases and 95% of malaria-related deaths. Countries such as Nigeria, the Democratic Republic of the Congo, Niger, and Tanzania reported over half of these deaths. Children under five years of age are particularly vulnerable, representing about 76% of all malaria deaths in the WHO African Region. Other high-risk groups include pregnant women and individuals with compromised immune systems.
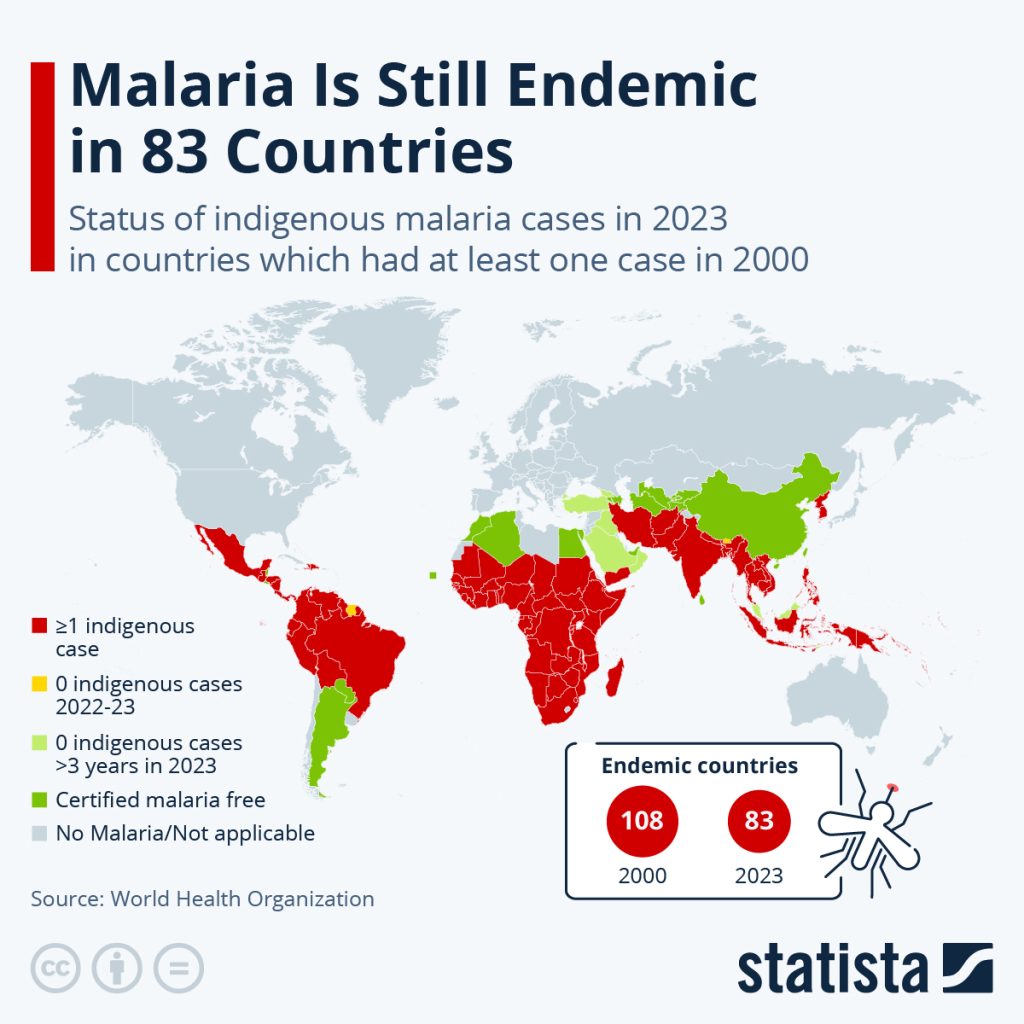
Figure 1: Status of malaria worldwide. [Image Description]
Malaria is transmitted through the bites of infected female Anopheles mosquitoes. Transmission is influenced by factors such as climate, rainfall, and human behavior, with peak transmission often occurring during and after rainy seasons. The high incidence of malaria places immense pressure on healthcare systems, particularly in resource-limited settings. Challenges include inadequate access to diagnostic tools, treatment, and preventive measures, as well as the emergence of drug-resistant malaria strains. Understanding the epidemiology of malaria is crucial for developing effective prevention and control strategies. While significant progress has been made, continued efforts are needed to reduce the global burden of this preventable and treatable disease.
2. The Plasmodium Parasite
Malaria parasites are eukaryotic microorganisms belonging to the genus Plasmodium. Five species infect humans – P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi. Among these, P. falciparum causes the most severe disease and highest mortality. The Plasmodium parasite has a complex life cycle (Figure 2) that alternates between the human host and the mosquito vector. In humans, the parasite passes through several stages. Sporozoites invade hepatocytes (liver cells) and undergo asexual multiplication, producing thousands of merozoites that enter the bloodstream. In the blood, merozoites infect red blood cells, multiply, and rupture the host cell to release more parasites, leading to the clinical symptoms of malaria. Some blood-stage parasites differentiate into gametocytes, which can be taken up by mosquitoes when they bite an infected human to continue the Plasmodium life cycle. Additional parasite stages in the mosquito host include ookinetes and oocysts, before eventual differentiation into sporozoites that make their way to mosquito salivary gland, from where they are delivered to the next human host during feeding.
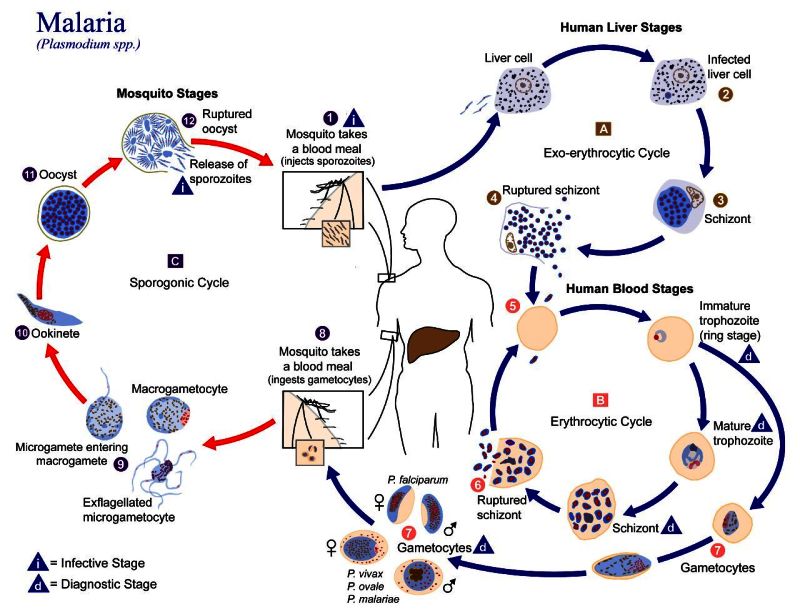
Figure 2. The Plasmodium parasite life cycle. The malaria parasite life cycle involves two hosts. During a blood meal, a malaria-infected female Anopheles mosquito inoculates sporozoites into the human host (1). Sporozoites infect liver cells (2) and mature into schizonts (3), which rupture and release merozoites (4). Of note, in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later. After this initial replication in the liver (exo-erythrocytic schizogony, A), the parasites undergo asexual multiplication in the erythrocytes (erythrocytic schizogony, B). Merozoites infect red blood cells (5). The ring stage trophozoites mature into schizonts, which rupture releasing merozoites (6). Some parasites differentiate into sexual erythrocytic stages (gametocytes, 7). Blood stage parasites are responsible for the clinical manifestations of the disease.
The gametocytes, male (microgametocytes) and female (macrogametocytes), are ingested by an Anopheles mosquito during a blood meal (8). The parasites’ multiplication in the mosquito is known as the sporogonic cycle C. While in the mosquito’s stomach, the microgametes penetrate the macrogametes generating zygotes (9). The zygotes in turn become motile and elongated (ookinetes, 10) which invade the midgut wall of the mosquito where they develop into oocysts (11). The oocysts grow, rupture, and release sporozoites (12), which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites (1) into a new human host perpetuates the malaria life cycle. [Image Description]
Plasmodium relies on host resources for survival. In humans, this includes scavenging hemoglobin and other nutrients from red blood cells. Plasmodium metabolic pathways are adapted to low-oxygen environments and include unique organelles, such as the apicoplast, which is essential for fatty acid and isoprenoid synthesis. These specialized metabolic processes make Plasmodium a target for many antimalarial drugs.
Alternating between hosts means that the parasite is constantly adapting to two very different environments: the mosquito and the human. From a systems biology perspective, Plasmodium’s ability to coordinate gene expression, metabolism, and immune evasion across these stages exemplifies complex network behavior.
3. Clinical Manifestations of Malaria in Humans
As we showed above, after a bite from an infected Anopheles mosquito, sporozoites enter the bloodstream and travel to the liver. The infection progresses through two stages. In the liver stage (asymptomatic, 7-14 days), parasites multiply silently inside hepatocytes and the infected individual usually experiences no symptoms. In P. vivax and P. ovale, dormant liver forms (hypnozoites) can remain for months or years before reactivating. In the blood stage (symptomatic), parasites emerge from the liver and infect red blood cells (RBCs). They replicate inside RBCs and rupture them in cycles of approximately 48-72 hours, depending on the species, causing the characteristic fever cycles of malaria.
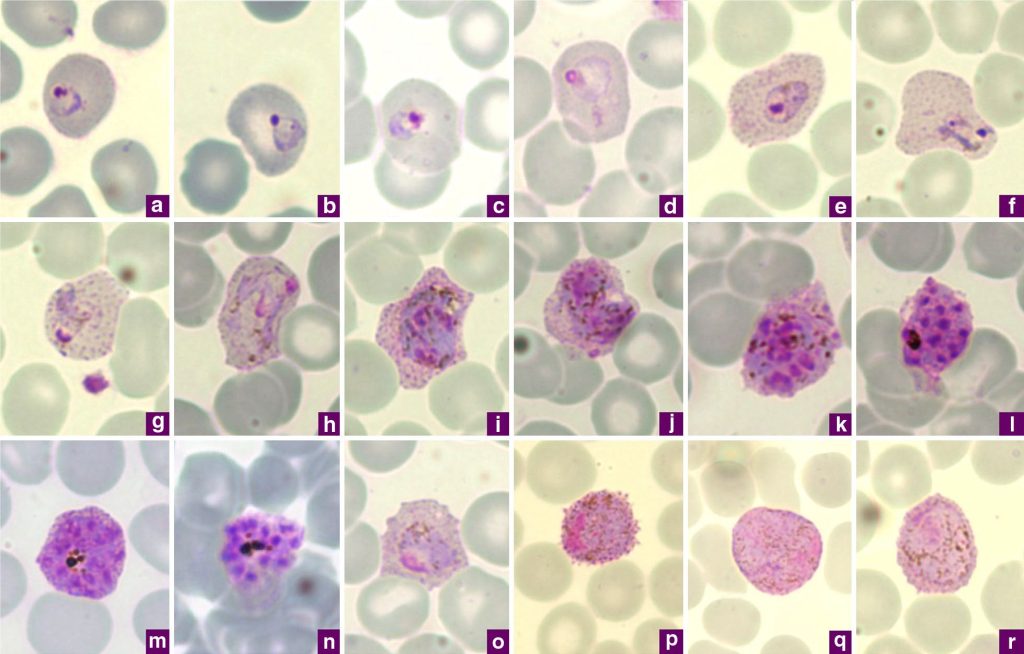
Figure 3: Microphotographs of Plasmodium vivax in Giemsa-stained thin blood films collected in Singapore. a, b) ring stages, c-e) young trophozoites, f-h) amoeboid trophozoites, i) young schizont, j-l) growing schizonts, m) developed schizont, n) mature schizont, o) young gametocyte, p) macrogametocyte, q, r) microgametocytes. [Image Description]
Symptoms of malaria reflect the body’s immune response to infected blood cells and include: recurrent fevers and chills, often in a cyclical pattern; sweating, headache, and fatigue; muscle aches and malaise; and nausea and vomiting in some cases. Severe malaria, most often caused by P. falciparum, can lead to severe anemia due to destruction of red blood cells; cerebral malaria, where parasites clog small blood vessels in the brain, causing seizures, confusion, or coma; and multi-organ failure, including kidney, liver, or respiratory complications.
Without prompt treatment, severe malaria can progress rapidly and be fatal within hours to days, especially in young children, pregnant women, and immunocompromised individuals. Death from malaria usually results from the body’s response to massive parasite replication and destruction of red blood cells. RBC destruction leads to anemia and hypoxia in tissues, impairing oxygen delivery. In P. falciparum infections, infected RBCs stick to vessel walls, causing blockage in small blood vessels and organ dysfunction. High parasite loads trigger an overwhelming immune response, which can exacerbate tissue damage.
From a systems biology standpoint, malaria symptoms emerge from interactions across scales: parasite replication, host immune response, red blood cell dynamics, and tissue oxygenation. Modeling these interactions helps predict disease severity, evaluate interventions, and understand why some individuals experience mild illness while others develop life-threatening complications.
4. Antimalarial Drugs: Mechanisms of Action
Antimalarial drugs target the Plasmodium parasite at different stages of its life cycle, disrupting key biological processes necessary for parasite survival and replication. Understanding these mechanisms illustrates how systems-level interventions can interrupt disease dynamics.
Targeting the Liver Stage
Some drugs act during the liver stage, when parasites infect hepatocytes:
- Primaquine targets dormant liver forms (hypnozoites) of P. vivax and P. ovale, preventing relapses. Its mechanism involves creating reactive oxygen species (such as hydrogen peroxide), which damage enzymes in the parasite.
Targeting the Blood Stage
Most antimalarial drugs act on the parasite during its blood stage, when it infects red blood cells and multiplies rapidly:
- Chloroquine interferes with the parasite’s digestion of hemoglobin. Normally, Plasmodium breaks down hemoglobin to obtain amino acids, releasing potentially toxic (to the parasite) heme in the process. The parasite detoxifies heme by converting it into hemozoin. Chloroquine inhibits this conversion, leading to accumulation of toxic heme in the parasite and parasite death.
- Artemisinin and derivatives generate reactive oxygen species (ROS) inside the parasite after activation by iron in heme, causing widespread damage to the parasite’s proteins and membranes. Artemisinins act very quickly and are often used in combination therapies to prevent resistance.
- Sulfadoxine-pyrimethamine inhibits sequential enzymes in the folate biosynthesis pathway, which the parasite uses to produce nucleotides for DNA replication. Disruption of folate metabolism halts parasite proliferation.
By targeting different stages of the life cycle, antimalarial drugs can reduce parasite load, prevent disease progression, and limit transmission. Combining drugs with complementary mechanisms (e.g., artemisinin-based combination therapies, ACTs) exemplifies a network-level intervention: it reduces the likelihood of resistance emerging and enhances the robustness of treatment outcomes across heterogeneous patient populations. When considering drug treatment strategies, it is also important to note that parasites, like bacteria, can evolve resistance to at least some drugs. Resistance to all of the major drug classes has been seen at one time or another in Plasmodium strains, which can complicate treatment.
5. The Mosquito Vector
Malaria parasites are transmitted to humans by the bite of female mosquitoes of the genus Anopheles. Not all mosquito species can transmit malaria; only Anopheles mosquitoes have the right physiology to support Plasmodium development. Figure 3 shows that in the mosquito midgut, ingested gametocytes fuse and develop into motile ookinetes, which then become oocysts. These oocysts release sporozoites that migrate to the mosquito’s salivary glands, ready to infect another human during the next bite.
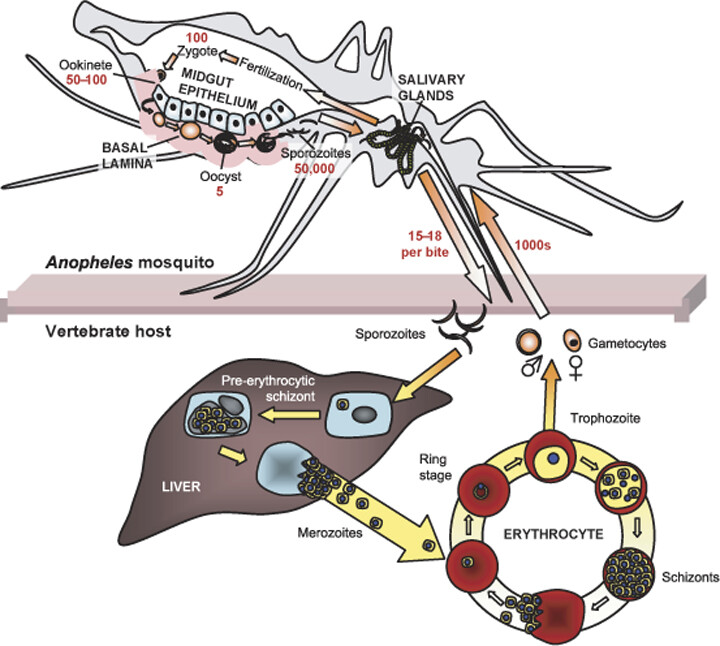
Figure 4. The Plasmodium life cycle in the mosquito host. Gametocytes are acquired by the mosquito upon blood-feeding. Within minutes, gametocytes develop into gametes that fuse to form a zygote. After approximately 24 hours, the motile ookinete invades the midgut epithelium and, upon reaching the basal lamina, differentiates into an oocyst. After approximately 16 days, the oocyst ruptures, releasing thousands of sporozoites into the haemocoel (gray). The sporozoites then migrate to the salivary glands and enter the salivary duct lumen from where they are injected into the vertebrate host (e.g., human) upon the next blood-feeding. The average number of Plasmodium parasites at each developmental stage (red) and host tissues (upper case) are shown. [Image Description]
The mosquito vector represents another layer of complexity. Its immune system, microbiome, and metabolic state influence whether Plasmodium can survive and complete its development. Environmental factors, such as temperature and humidity, also affect mosquito lifespan and feeding behavior, which in turn shape transmission dynamics. Scientists have noted that changes to climate could result in expansion of the geographic range of the Anopheles mosquito, potentially exposing more human populations to malaria transmission.
6. Population-Level Public Health Interventions for Malaria
Effective malaria control usually includes interventions that act not only at the individual level (e.g., drugs) but across populations and ecosystems. Systems biology perspectives emphasize the interactions between human, parasite, and mosquito populations, as well as environmental factors. A variety of examples are shown below. It should be noted that none of these strategies will be 100% effective as they are implemented in the real world, and how effective they are alone or in combination will be important considerations in how, where, and when they are chosen.
Vector Control
Sleeping under insecticide-treated bed nets (ITNs) protects individuals from mosquito bites and reduces mosquito survival, lowering transmission at the community level. Indoor residual spraying (IRS) involves spraying insecticides on walls and ceilings, and it kills mosquitoes resting indoors, interrupting the life cycle. However, some Anopheles mosquito populations have evolved resistance to insecticides, complicating both ITN and IRS strategies. Draining standing water, modifying irrigation, or improving housing infrastructure reduces mosquito breeding sites.
Chemoprevention
Monthly doses of antimalarial drugs for children in high-transmission regions during rainy seasons significantly decrease clinical cases and deaths. Similarly, pregnant women receive antimalarials at scheduled intervals to protect both mother and fetus from malaria’s severe effects.
Rapid Diagnosis and Treatment
Ensuring access to diagnostic tools (rapid diagnostic tests, RDTs) and effective therapy reduces the parasite reservoir in humans, lowering transmission potential. Meanwhile, first-line treatment with Artemisinin-based combination therapies (ACTs) reduces parasite load quickly and helps prevent the spread of drug-resistant strains.
Surveillance and Data-Driven Strategies
Monitoring malaria incidence, drug resistance, and mosquito populations enables targeted interventions. These data may be used to build predictive models, which can anticipate outbreaks, optimize resource allocation, and evaluate the impact of combined interventions on population-level outcomes.
Vaccination
Vaccination programs, combined with other interventions, contribute to lowering the disease burden at the population level. The RTS,S/AS01 malaria vaccine, now recommended for children in high-transmission areas, reduces the risk of severe malaria.
The most effective malaria control usually involves multi-layered interventions: targeting the parasite (drugs), vector (mosquito control), human susceptibility (vaccines, chemoprevention), and environmental factors. Modeling these interactions helps public health authorities predict the effects of interventions, identify synergistic strategies, and reduce the overall burden of disease.
7. A Systems Biology View of Malaria
Malaria is not just a parasite infection – it is a dynamic system involving three key players: the human host, the Plasmodium parasite, and the Anopheles mosquito. Each part influences the others through feedback loops, population dynamics, and ecological constraints. Systems biology approaches can help us understand malaria at multiple levels. Systems biologists can perform network modeling of host-parasite and parasite-mosquito interactions, build population models linking human infection rates to mosquito ecology, and implement pmics technologies reveal how the parasite’s gene expression changes during its life cycle. By combining these approaches, researchers can identify critical nodes or weak points in the malaria system – targets for drugs, vaccines, or vector control measures.
Practice Questions
Image Descriptions
Figure 1: The image is a world map illustrating the status of indigenous malaria cases in 83 countries for the year 2023. The map categorizes countries using color codes: red for countries with one or more indigenous cases, yellow for no indigenous cases from 2022-23, green for no indigenous cases for over three years, dark green for certified malaria-free countries, and gray for areas where malaria is not applicable. The map notably highlights that most endemic countries are located in Africa, Southeast Asia, and parts of South America. There is a white box with two red circles indicating the number of endemic countries: 108 in 2000 and 83 in 2023. A mosquito icon is included near this box. [Return to Figure]
Figure 2:
The image is an infographic illustrating the life cycle of the malaria parasite, Plasmodium spp., involving both mosquito and human stages. It is organized into distinct cycles: mosquito stages, human liver stages, and human blood stages, with specific focus on infective and diagnostic stages. The diagram is circular, with arrows indicating the sequence of stages.
In the mosquito stages, it starts with a mosquito taking a blood meal, injecting sporozoites into a human host. The sporozoites travel to the liver, initiating the exo-erythrocytic cycle. Here, liver cells become infected, forming schizonts, which eventually rupture.
Moving to the human blood stages, merozoites infect red blood cells, leading to the erythrocytic cycle. This includes the development from immature trophozoites to mature trophozoites, and finally, to schizonts, which also rupture, releasing more merozoites. Some merozoites develop into gametocytes, which can be taken up by mosquitoes.
In mosquitoes, the gametocytes develop through stages such as ookinete and oocyst, completing the sporogonic cycle with the release of sporozoites back into another human host.
The diagram uses color coding and labels to differentiate each stage, and includes symbols indicating infective and diagnostic stages.
[Return to Figure]
Figure 3: The image displays a grid of eighteen microscopic images labeled from “a” to “r.” Each image shows blood cells with various structures and characteristics prominently stained in different shades of purple. The background in each image is a pale, translucent color, allowing the blood cells and distinctive structures to stand out. The cells vary in size and texture, with some appearing smooth while others have more complex, granular interiors. The labeling of each image is in a small purple box located at the bottom corner of each segment. [Return to Figure]
Figure 4: The image is a detailed diagram illustrating the life cycle of the malaria parasite, specifically within the Anopheles mosquito and a vertebrate host. At the top, a cross-section of the mosquito shows labeled anatomical features like the midgut epithelium and salivary glands. The journey of the malaria parasite begins with gametocytes forming a zygote in the mosquito’s gut, transitioning into an ookinete, and then developing into oocysts on the basal lamina, with each stage quantified by numbers. Sporozoites multiply to significant numbers before migrating to the salivary glands. Below, the diagram shows the transmission of sporozoites to a vertebrate host during a mosquito bite. Inside the host’s liver, sporozoites develop into pre-erythrocytic schizonts and merozoites. The cycle concludes in the bloodstream within erythrocytes, transitioning between ring stages, trophozoites, and schizonts, before releasing more merozoites. [Return to Figure]
Licenses and Attributions
“Malaria as a Systems Biology Challenge” was partially written by ChatGPT 5, with prompting, fact-checking, editing, and supplementation by Craig Stephens. “Malaria as a Systems Biology Challenge” is licensed under CC BY-NC 4.0 except where otherwise noted. Section 1 of this chapter is adapted from the “World malaria report 2023” by the World Health Organization used under CC BY-NC-SA 3.0. Section 1 of “Malaria as a Systems Biology Challenge” is licensed under CC BY-NC-SA 4.0.
Media Attributions
- 1C-O-1.1 Figure – malaria geography © Katharina Buchholz is licensed under a CC BY-ND (Attribution NoDerivatives) license
- 1C-O-1.2 Figure – malaria lifecycle © Centers for Disease Control is licensed under a Public Domain license
- 1C-O-1.3 Figure – microphotographs_of_Plasmodium_vivax_in_Giemsa-stained_thin_blood_films © Chavatte JM, Tan SB, Snounou G, Lin RT is licensed under a CC BY (Attribution) license
- 1C-O-1.5 Figure – malaria lifecycle in mosquito © Anthony E. Brown & Flaminia Catteruccia is licensed under a CC BY (Attribution) license
