4.4 Active Membrane Transport
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe how electrochemical gradients affect ions.
- Distinguish between primary active transport and secondary active transport.
Active transport mechanisms require the cell’s energy, usually in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient—that is, if the substance’s concentration inside the cell is greater than its concentration in the extracellular fluid (and vice versa)—the cell must use energy to move the substance. Some active transport mechanisms move small-molecular weight materials, such as ions, through the membrane. Other mechanisms transport much larger molecules.
Electrochemical Gradient
We have discussed simple concentration gradients—a substance’s differential concentrations across a space or a membrane—but in living systems, gradients are more complex. Because ions move into and out of cells and because cells contain proteins that do not move across the membrane and are mostly negatively charged, there is also an electrical gradient, a difference of charge, across the plasma membrane. The interior of living cells is electrically negative with respect to the extracellular fluid in which they are bathed, and at the same time, cells have higher concentrations of potassium (K+) and lower concentrations of sodium (Na+) than the extracellular fluid. Thus, in a living cell, the concentration gradient of Na+ tends to drive it into the cell, and its electrical gradient (a positive ion) also drives it inward to the negatively charged interior. However, the situation is more complex for other elements such as potassium. The electrical gradient of K+, a positive ion, also drives it into the cell, but the concentration gradient of K+ drives K+ out of the cell (Figure 4.4.1). We call the combined concentration gradient and electrical charge that affects an ion its electrochemical gradient.
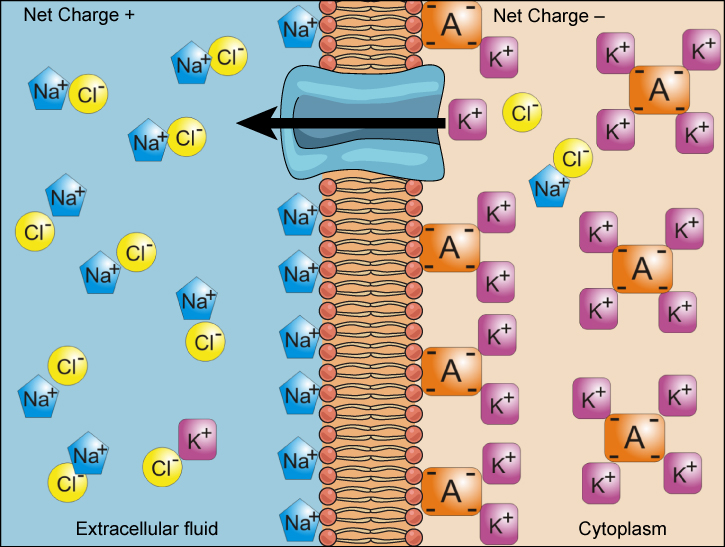
Moving Against a Gradient
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy comes from ATP generated through the cell’s metabolism. Active transport mechanisms, or pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances that living cells require in the face of these passive movements. A cell may spend much of its metabolic energy supply maintaining these processes. (A red blood cell uses most of its metabolic energy to maintain the imbalance between exterior and interior sodium and potassium levels that the cell requires.) Because active transport mechanisms depend on a cell’s metabolism for energy, they are sensitive to many metabolic poisons that interfere with the ATP supply.
Two mechanisms exist for transporting small-molecular weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport does not directly require ATP: instead, it is the movement of material due to the electrochemical gradient established by primary active transport.
Carrier Proteins for Active Transport
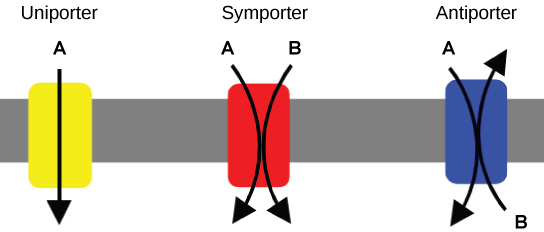
An important membrane adaptation for active transport is the presence of specific carrier proteins or pumps to facilitate movement: there are three protein types or transporters (Figure 4.4.2). A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. An antiporter also carries two different ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules like glucose. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-K+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca2+ ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
Primary Active Transport
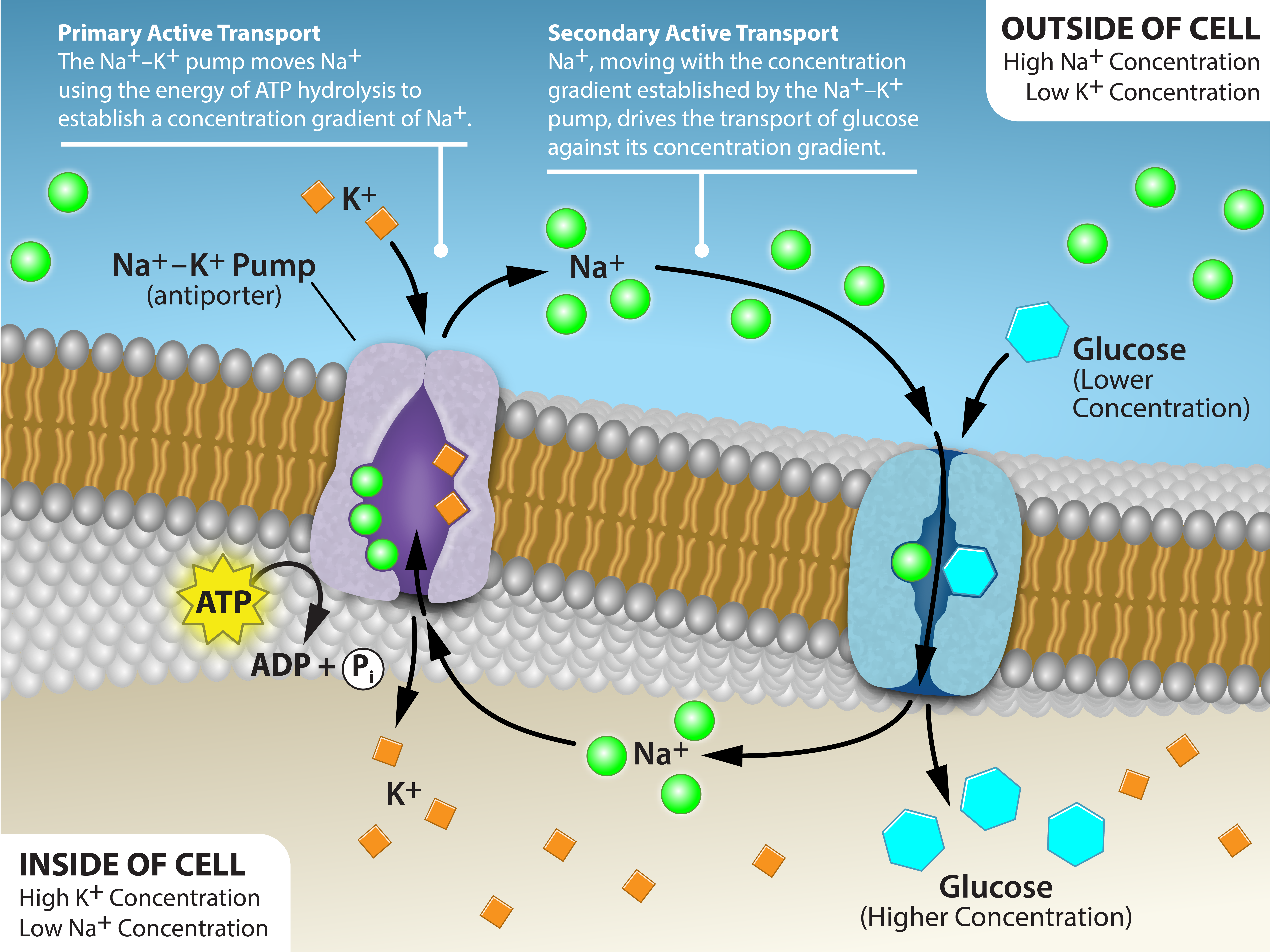
The primary active transport that functions with the active transport of sodium and potassium allows secondary active transport to occur. The second transport method is still active because it depends on using energy as does primary transport (Figure 4.4.3).
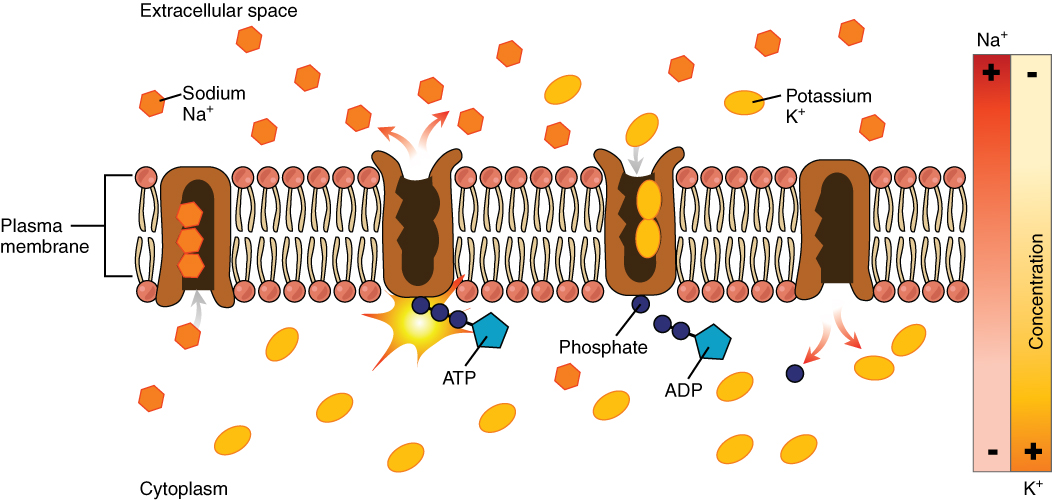
One of the most important pumps in animal cells is the sodium-potassium pump (Na+-K+ ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na+ and K+) in living cells. The sodium-potassium pump moves K+ into the cell while moving Na+ out at the same time, at a ratio of three Na+ for every two K+ ions moved in. The Na+-K+ ATPase exists in two forms, depending on its orientation to the cell’s interior or exterior and its affinity for either sodium or potassium ions.
Several things have happened as a result of this process. At this point, there are more sodium ions outside the cell than inside and more potassium ions inside than out. For every three sodium ions that move out, two potassium ions move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Secondary Active Transport
Secondary active transport uses the kinetic energy of the sodium ions to bring other compounds, against their concentration gradient into the cell. As sodium ion concentrations build outside of the plasma membrane because of the primary active transport process, this creates an electrochemical gradient. If a channel protein exists and is open, the sodium ions will move down its concentration gradient across the membrane. This movement transports other substances that must be attached to the same transport protein in order for the sodium ions to move across the membrane (Figure 4.4.4). Many amino acids, as well as glucose, enter a cell this way. This secondary process also stores high-energy hydrogen ions in the mitochondria of plant and animal cells in order to produce ATP. The potential energy that accumulates in the stored hydrogen ions translates into kinetic energy as the ions surge through the channel protein ATP synthase, and that energy then converts ADP into ATP.
Practice Questions
Glossary
electrochemical gradient
the combined concentration gradient and electrical charge that affects an ion’s movement across a membrane.
primary active transporter
harnesses the energy of ATP hydrolysis to drive ions and small polar molecules across the membrane against their concentration gradient.
pump protein
integral membrane protein that actively promotes the movement of ions and small polar substances across the membrane from areas of low concentration to areas of high concentration.
secondary active transporter
uses the kinetic energy of ions moving down their concentration gradient to actively move other compounds, against their concentration gradient.
Figure Descriptions
Figure 4.4.1. The image illustrates a cell membrane separating two regions labeled “Extracellular fluid” on the left and “Cytoplasm” on the right. The membrane is depicted as a double layer, with a central blue channel allowing for ion passage. Various ions are represented as colored shapes with labels: Na+ (sodium ions) as blue hexagons, Cl- (chloride ions) as yellow circles, K+ (potassium ions) as pink squares, and A- (anions) as orange squares. In the extracellular fluid, Na+ and Cl- ions predominate, resulting in a net positive charge. In the cytoplasm, K+ and A- ions dominate, contributing to a net negative charge. An arrow indicates ion movement from the extracellular fluid to the cytoplasm through the channel. [Return to Figure 4.4.1]
Figure 4.4.2. The image illustrates three different types of membrane transport proteins: a uniporter, symporter, and antiporter. Each transport type is represented by a colored rectangle embedded in a gray horizontal membrane. The uniporter on the left is shown in yellow with a single straight arrow labeled “A” passing directly downward through it, indicating one type of substrate moves through in one direction. The symporter in the middle is depicted in red with two curved arrows labeled “A” and “B” entering from above and moving downward through the membrane in the same direction, representing the simultaneous transport of two different substrates. The antiporter on the right is in blue, with two curved arrows labeled “A” and “B” crossing as they pass through the membrane in opposite directions, indicating the exchange of substrates. [Return to Figure 4.4.2]
Figure 4.4.3. The image illustrates a cross-section of a cell’s plasma membrane, depicting the sodium-potassium pump mechanism. The membrane is composed of a double layer of phospholipids, illustrated as brown lines with round, pink heads facing outward. Three large protein structures, depicted in brown color, are integrated into the membrane. Sodium ions, represented as orange hexagons marked “Na+,” and potassium ions, shown as yellow ovals marked “K+,” are seen on either side of the membrane. Arrows indicate the movement of ions: sodium ions are moving out of the cell, while potassium ions are moving into the cell. The sunburst symbol represents ATP conversion to ADP, with associated labels and phosphate. A color gradient bar on the right illustrates the concentration levels of Na+ and K+ with red indicating high concentration and light yellow indicating low concentration. Labels clearly identify different components of the image. [Return to Figure 4.4.3]
Figure 4.4.4. The image illustrates a cell’s phospholipid bilayer with two key transporters. On the left, a purple Na⁺–K⁺ pump (antiporter) hydrolyzes ATP (depicted as a yellow star) to eject three green sodium ions (Na+) outside the cell and bring two orange potassium ions (K+) inside, establishing high external Na⁺ and high internal K⁺. The transcribed text states ATP → ADP + Pᵢ. Curved black arrows and the transcribed texts of 1. Primary Active Transport; The Na⁺–K⁺ pump moves Na⁺ using the energy of ATP hydrolysis to establish a concentration gradient of Na⁺. and 2. Secondary Active Transport: Na⁺, moving with the concentration gradient established by the Na⁺–K⁺ pump, drives the transport of glucose against its concentration gradient. highlight this energy-dependent step. On the right, a blue Na⁺/glucose symporter exploits the resulting sodium gradient: one Na⁺ ion moving down its electrochemical gradient passes through the carrier together with a cyan hexagonal glucose molecule, which is dragged against its own concentration gradient into the cytoplasm, an example of secondary active transport (co-transport). Two boxes at the corners with transcribed text 1. Outside of cell: High Na⁺ Concentration, Low K⁺ Concentration and 2. Inside of cell: High K⁺ Concentration, Low Na⁺ Concentration emphasizes the contrasting ion concentrations inside versus outside the cell, and arrow paths connect the pump’s action with the symporter, illustrating how the energy stored in the Na⁺ gradient powers glucose uptake. [Return to Figure 4.4.4]
Licenses and Attributions
“4.4 Active Membrane Transport” is adapted from “5.3 Active Transport” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “4.4 Active Membrane Transport” is licensed under CC-BY-NC 4.0.
Media Attributions
- 1A.B.8 Electrochemical gradient © Synaptitude/Wikimedia is licensed under a CC BY (Attribution) license
- 1A.B.8 Carrier Proteins © Lupask/Wikimedia Commons is licensed under a CC BY (Attribution) license
- 1A.B.8_Sodium_Potassium_Pump © OpenStax Anatomy and Physiology is licensed under a CC BY (Attribution) license
- 1A.B.8 Na_Glucose cotransporter © Rao, A., Ryan, K., Tag, A. and Fletcher, S. is licensed under a CC BY (Attribution) license
