3.2 Carbohydrate Structure and Function
Learning Objectives
By the end of this section, you will be able to do the following:
- Explain the role of carbohydrates in cells and in the extracellular materials of animals and plants.
- Explain the functions of common monosaccharides, disaccharides, and polysaccharide.
- Differentiate between the structures of common monosaccharides, disaccharides, and polysaccharides.
Most people are familiar with carbohydrates, one type of macromolecule, especially when it comes to what we eat. To lose weight, some individuals adhere to “low-carb” diets. Athletes, in contrast, often “carb-load” before important competitions to ensure that they have enough energy to compete at a high level. Carbohydrates are, in fact, an essential part of our diet. Grains, fruits, and vegetables are all natural carbohydrate sources that provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple foods. Carbohydrates also have other important functions in humans, animals, and plants.
Molecular Structures
The stoichiometric formula (CH2O)n, where n is the number of carbons in the molecule represents carbohydrates. In other words, the ratio of carbon to hydrogen to oxygen is 1:2:1 in carbohydrate molecules. This formula also explains the origin of the term “carbohydrate”: the components are carbon (“carbo”) and the components of water (hence, “hydrate”). Scientists classify carbohydrates into three subtypes: monosaccharides, disaccharides, and polysaccharides. Of those, only disaccharides and polysaccharides are considered macromolecules as they are polymers of different forms of monosaccharides.
Monosaccharides
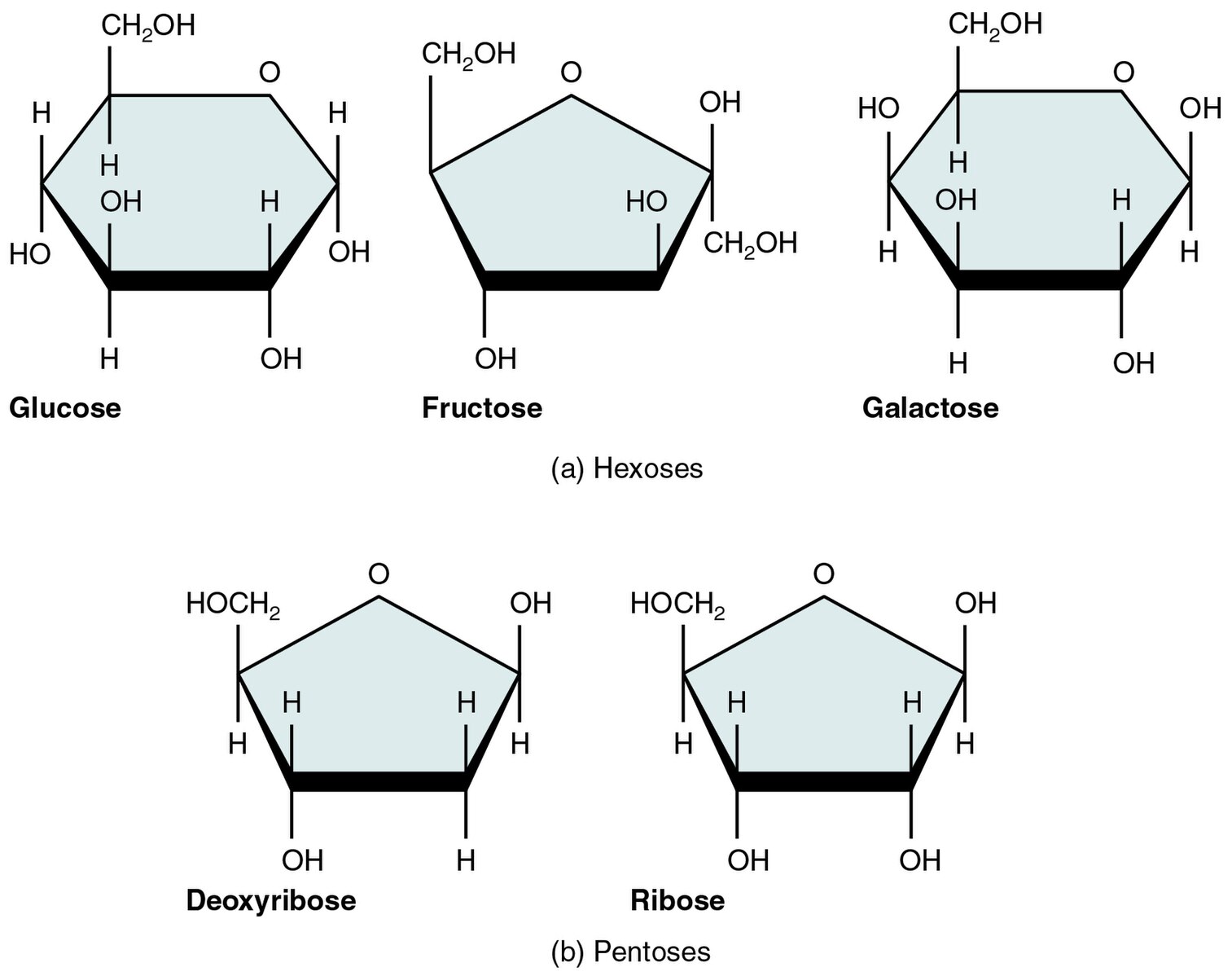
Monosaccharides (mono- = “one”; sacchar- = “sweet”) are simple sugars, the most common of which is glucose. In monosaccharides, the number of carbons usually ranges from three to seven. Most monosaccharide names end with the suffix -ose. If the sugar has an aldehyde group (the functional group with the structure R-CHO), it is an aldose, and if it has a ketone group (the functional group with the structure RC(=O)R’), it is a ketose. Depending on the number of carbons in the sugar, they can be trioses (three carbons), pentoses (five carbons), and/or hexoses (six carbons). Figure 3.2.1 illustrates some common monosaccharides.
The chemical formula for glucose is C6H12O6. In humans, glucose is an important source of energy. During cellular respiration, energy releases from glucose, and that energy helps make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water, and glucose in turn provides energy requirements for the plant. Humans and other animals that feed on plants often obtain glucose from catabolized (cell breakdown of larger molecules) starch.
Galactose (part of lactose, or milk sugar) and fructose (found in sucrose, in fruit) are other common monosaccharides. Although glucose, galactose, and fructose all have the same chemical formula (C6H12O6), they differ structurally and chemically (and are isomers) because of the different arrangement of functional groups around the asymmetric carbon.
Disaccharides
Disaccharides (di- = “two”) form when two monosaccharides undergo a dehydration reaction (or a condensation reaction or dehydration synthesis). During this process, one monosaccharide’s hydroxyl group combines with another monosaccharide’s hydrogen, releasing a water molecule and forming a covalent bond. A covalent bond forms between a carbohydrate molecule and another molecule (in this case, between two monosaccharides) (Figure 3.2.2).
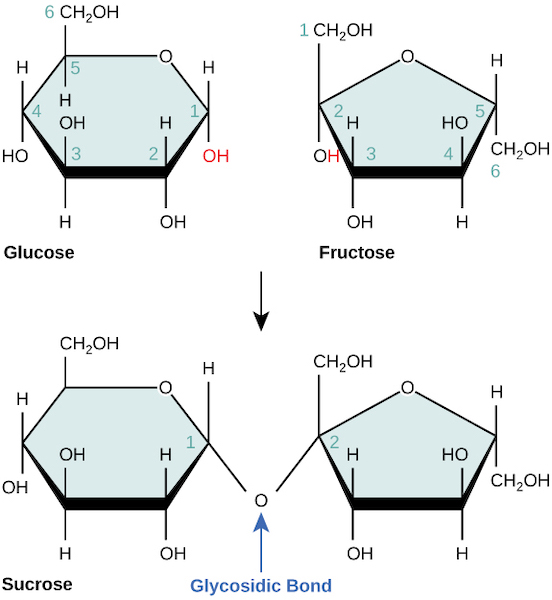
Common disaccharides include lactose, maltose, and sucrose. Lactose is a disaccharide consisting of the monomers glucose and galactose. It is naturally in milk. Maltose, or malt sugar, is a disaccharide formed by a dehydration reaction between two glucose molecules. The most common disaccharide is sucrose, or table sugar, which is comprised of glucose and fructose monomers.
Polysaccharides: Complex Carbohydrates
A long chain of monosaccharides linked by covalent bonds called (glycosidic bonds) is a polysaccharide (poly- = “many”). The chain may be branched or unbranched, and it may contain different types of monosaccharides. The molecular weight may be 100,000 daltons or more depending on the number of joined monomers. starch, glycogen, cellulose, and chitin are primary examples of polysaccharides.
Plants store sugars in the form of starch. In plants, an amylose and amylopectin mixture (both glucose polymers) comprise these sugars. Plants are able to synthesize glucose, and they store the excess glucose, beyond their immediate energy needs, as starch in different plant parts, including roots and seeds. The starch in the seeds provides food for the embryo as it germinates and can also act as a food source for humans and animals. Enzymes break down the starch that humans consume. For example, an amylase present in saliva catalyzes the breakdown of this starch into smaller molecules, such as maltose and glucose. The cells can then absorb the glucose.
Glucose starch comes in two forms, amylose and amylopectin. As Figure 3.2.3 illustrates, unbranched glucose monomer chains form starch; whereas, amylopectin is a branched polysaccharide. Glycogen is the storage form of glucose in humans and other vertebrates and is comprised of monomers of glucose. Glycogen is the animal equivalent of starch and is a highly branched molecule usually stored in liver and muscle cells. Whenever blood glucose levels decrease, glycogen breaks down to release glucose. Cellulose is the most abundant natural biopolymer. The cell walls around plant cells are mostly comprised of cellulose. This provides the cell structural support. Wood and paper are mostly cellulosic in nature.
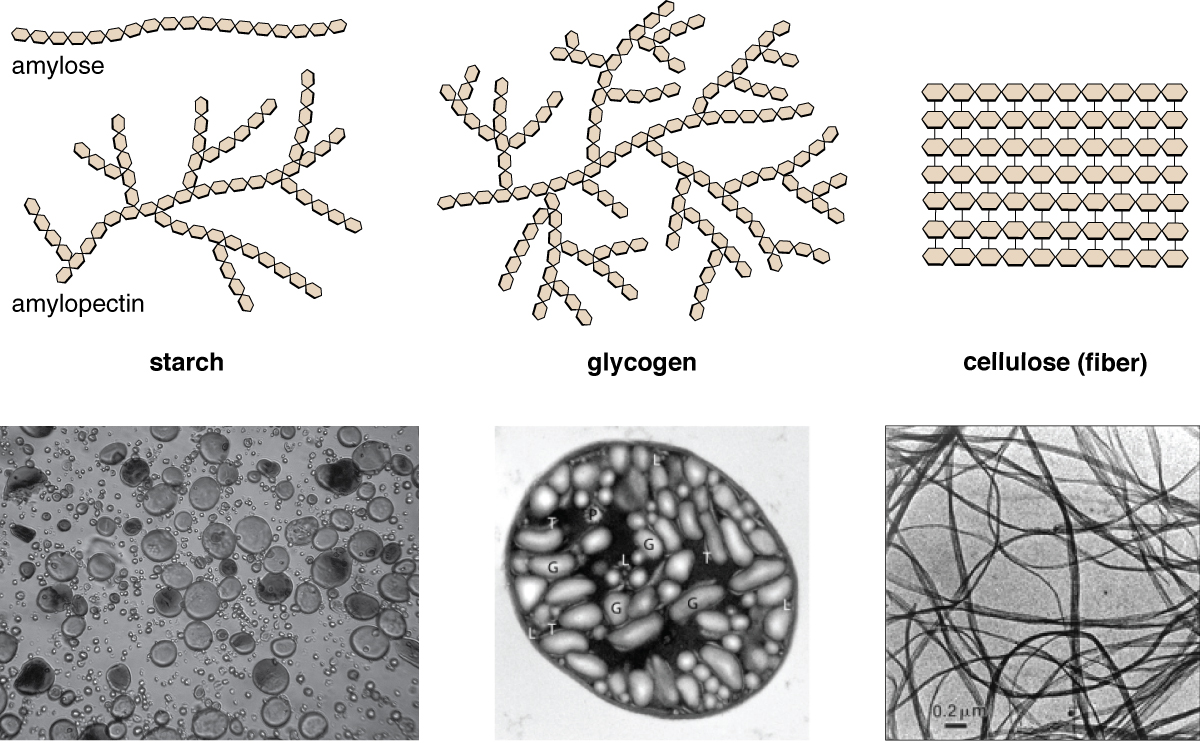
As Figure 3.2.3 shows, every other glucose monomer in cellulose is flipped over, and the monomers are packed tightly as extended long chains. This gives cellulose its rigidity and high tensile strength—which is so important to plant cells. While human digestive enzymes cannot break down the covalent bonds between glucose molecules in cellulose, herbivores such as cows, koalas, and buffalos are able, with the help of the specialized bacteria in their stomach, to digest plant material that is rich in cellulose and use it as a food source. In some of these animals, certain species of bacteria and protists reside in the rumen (part of the herbivore’s digestive system) and secrete the enzyme cellulase. The appendix of grazing animals also contains bacteria that digest cellulose, giving it an important role in ruminants’ digestive systems. Cellulases can break down cellulose into glucose monomers that animals use as an energy source. Termites are also able to break down cellulose because of the presence of other organisms in their bodies that secrete cellulases.
Carbohydrates serve various functions in different animals. Arthropods (insects, crustaceans, and others) have an outer skeleton, the exoskeleton, which protects their internal body parts. This exoskeleton is made of the biological macromolecule chitin, which is a nitrogen-containing polysaccharide. It is made of repeating N-acetyl-β-d-glucosamine units, which are a modified sugar. Chitin is also a major component of fungal cell walls.
Video 3.2.1. Biomolecules: The Carbohydrates by Wisc-Online
Practice Questions
Glossary
monosaccharide
the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
disaccharide
the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
polysaccharide
long chain of monosaccharides linked by glycosidic bonds.
starch
complex carbohydrate made up of glucose subunits found in plants. Used to store energy.
glycogen
complex carbohydrate made up of glucose subunits found in animals. Used to store energy.
cellulose
complex carbohydrate made up of glucose subunits found in plants. The main component of a plant’s cell wall, which provides structural support.
Figure Descriptions
Figure 3.2.1. The image depicts two groups of simple sugar molecules: hexoses and pentoses, shown in chemical structural diagrams with each molecule represented by a shaded polygon. The top row features three six-carbon hexoses: Glucose, Fructose, and Galactose. Each hexose is drawn as a hexagon with specific atomic labels including oxygen (O), hydrogen (H), and hydroxyl groups (OH) attached at various positions. Glucose and Galactose are labeled with CH2OH at the top right vertex, whereas Fructose shows it at the top left. The bottom row presents two five-carbon pentoses: Deoxyribose and Ribose. Both are pentagons with similar atomic labels, including a top vertex marked with HOCH2. The diagrams use straight lines for bonds and indicate molecular structure and orientation of each sugar type. Labels beneath each molecule serve to identify them. [Return to Figure 3.2.1]
Figure 3.2.2. The image illustrates the chemical structure of glucose, fructose, and sucrose with a focus on the glycosidic bond formation. At the top left, a hexagonal ring structure represents glucose, with various carbon and hydroxyl groups labeled numerically in black and light blue. To the top right, fructose is displayed with a similar hexagonal ring structure, also labeled with carbon and hydroxyl groups. Both molecules show CH(_2)OH and OH groups extending from the rings. An arrow points downward to the bottom structures, indicating a reaction or transformation. Below, the structures of sucrose are shown, with glucose and fructose linked via an oxygen atom forming a glycosidic bond. The bond is highlighted with an arrow labeled “Glycosidic Bond” in blue. [Return to Figure 3.2.2]
Figure 3.2.3. The image is a composition of six sections illustrating the structural and microscopic differences among various polysaccharides: starch, glycogen, and cellulose. The top row features schematic representations. On the left, “amylose” is depicted as a linear chain of hexagonal units. Next to it, “amylopectin” in starch is shown as a branched chain. The middle image shows “glycogen,” another branched chain but denser and more complex. On the right, “cellulose” appears as parallel linear chains of hexagonal units, suggesting a fibrous nature. The bottom row presents black-and-white microscopic images. The first shows granular structures, resembling small circles and ovals, labeled as starch. The second image highlights a detailed view with labeled sections (G, T, L, P), representing glycogen organization. The third image displays thin, intertwined fiber-like strands, illustrating cellulose structure. There is a scale bar labeled “0.2 µm” in the cellulose image. [Return to Figure 3.2.3]
Licenses and Attributions
“3.2 Carbohydrate Structure Function” is adapted from “3.2 Carbohydrates” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “3.2 Carbohydrate Structure Function” is licensed under CC-BY-NC 4.0.
Media Attributions
- 1A.B_Five_Important_Monosaccharides-01 © OpenStax College is licensed under a CC BY (Attribution) license
- 1A.B_sucrose_formation © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- 1A.B_polysacch © OpenStax Microbiology is licensed under a CC BY (Attribution) license
biological macromolecules made up of monosaccharides; serve as energy sources and structural support molecules.
the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
long chain of monosaccharides linked by glycosidic bonds
complex carbohydrate made up of glucose subunits found in plants. Used to store energy.
complex carbohydrate made up of glucose subunits found in animals. Used to store energy.
complex carbohydrate made up of glucose subunits found in plants. The main component of a plant's cell wall, which provides structural support.
a certain materials ability to maintain integrity while sustaining pulling force
