10.5 Metabolism without Oxygen
Learning Objectives
By the end of this section, you will be able to do the following
- Explain the fundamental difference between anaerobic cellular respiration and fermentation
- Describe the type of fermentation that readily occurs in animal cells and the conditions that initiate that fermentation
In aerobic respiration, the final electron acceptor is an oxygen molecule, O2. If aerobic respiration occurs, then ATP will be produced using the energy of high-energy electrons carried by NADH or FADH2 to the electron transport chain. If aerobic respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for the glycolytic pathway to continue. How is this done? Some living systems use an organic molecule as the final electron acceptor. Processes that use an organic molecule to regenerate NAfermentationD+ from NADH are collectively referred to as fermentation. In contrast, in some living systems, the electron transport chain (ETC) uses an inorganic molecule as a final electron acceptor, which is called anaerobic cellular respiration. Both processes allow organisms to convert energy for their use in the absence of oxygen. Both methods are anaerobic, in which organisms convert energy for their use in the absence of oxygen.
Anaerobic Cellular Respiration
Certain prokaryotes, including some species in the domains Bacteria and Archaea, use anaerobic respiration. For example, a group of archaeans called methanogens reduces carbon dioxide to methane to oxidize NADH. These microorganisms are found in soil and in the digestive tracts of ruminants, such as cows and sheep. Similarly, sulfate-reducing bacteria, most of which are anaerobic (Figure 10.5.1), reduce sulfate to hydrogen sulfide to regenerate NAD+ from NADH.
Lactic Acid and Alcohol Fermentation
Some of our cells as well as many other organisms, use the process of fermentation to regenerate NAD+ from NADH. There are many forms of fermentation across the tree of life. We will focus on two specific pathways:
- Alcohol fermentation is observed in yeast and responsible for the production of ethanol in wine and beer.
- Lactic acid fermentation takes place in certain bacteria (such as lactobacillus used to make yogurt) and in certain animal cells (e.g. muscle cells or red blood cells)
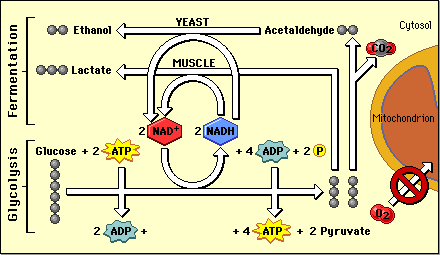
Fermentation reactions take place in the cytoplasm of cells. They are not sufficient to rescue the function of the mitochondrion where NAD+ has been depleted by the absence of O2. However, in all cases, they are able to regenerate enough NAD+ to keep glycolysis producing ATP as long as glucose is available. The products of fermentation, ethanol and lactic acid, can become toxic to cells if they build up so this form of anaerobic energy production becomes self-limiting.
Video 10.5.1. Fermentation by the Amoeba Sisters.
Practice Questions
Glossary
fermentation
processes that use an organic molecule to regenerate NAD+ from NADH
lactic acid
chemical your body produces when your cells break down carbohydrates for energy
aerobic respiration
process in which cells break down glucose using oxygen to release energy
anaerobic respiration
process in which cells break down glucose without oxygen to release energy
Licenses and Attributions
“10.5 Metabolism without Oxygen” is adapted from “7.5 Metabolism without Oxygen” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “10.5 Metabolism without Oxygen” is licensed under CC-BY-NC 4.0.
Media Attributions
- Alcohol_fermentation_process © McZusatz is licensed under a CC BY-SA (Attribution ShareAlike) license
processes that use an organic molecule to regenerate NAD+ from NADH
process in which cells break down glucose without oxygen to release energy
