8.2 Water’s Cohesive and Adhesive Properties
Learning Objectives
By the end of this section, you will be able to do the following:
- Define the properties of cohesive and adhesive forces
- Describe the relationship of surface tension to cohesion and capillary action to adhesion
- Explain how the properties of cohesion and adhesion support water transport in plants
Have you ever filled a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-gas (water-air) interface, although there is no more room in the glass.
Cohesion allows for surface tension, the capacity of a substance to withstand rupturing when placed under tension or stress. This is also why water forms droplets when on a dry surface rather than flattening by gravity. When we place a small scrap of paper onto a water droplet, the paper floats on top even though paper is denser (heavier) than the water. Cohesion and surface tension keep the water molecules’ hydrogen bonds intact and support the item floating on the top. It’s even possible to “float” a needle on top of a glass of water if you place it gently without breaking the surface tension, as Figure 8.2.1 shows.

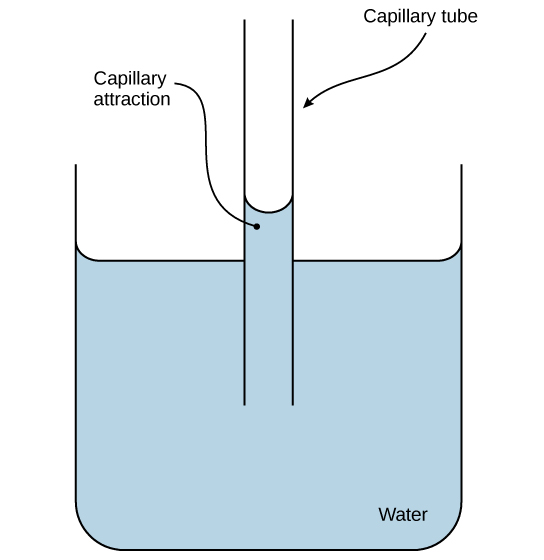
These cohesive forces are related to water’s property of adhesion, or the attraction between water molecules and other molecules. This attraction is sometimes stronger than water’s cohesive forces, especially when the water is exposed to charged surfaces such as those on the inside of thin glass tubes known as capillary tubes. We observe adhesion when water “climbs” up the tube placed in a glass of water: notice that the water appears to be higher on the tube’s sides than in the middle. This is because the water molecules are attracted to the capillary’s charged glass walls more than they are to each other and therefore adhere to it. We call this type of adhesion capillary action, as Figure 8.2.2 illustrates.
Why are cohesive and adhesive forces important for life? Cohesive and adhesive forces are important for transporting water from the roots to the leaves in plants. These forces create a “pull” on the water column. This pull results from the tendency of water molecules evaporating on the plant’s surface to stay connected to water molecules below them, and so they are pulled along. Plants use this natural phenomenon to help transport water from their roots to their leaves. Without these properties of water, plants would be unable to receive the water and the dissolved minerals they require. In another example, insects such as the water strider, as Figure 8.2.3 shows, use the water’s surface tension to stay afloat on the water’s surface layer and even mate there.

Practice Questions
Glossary
cohesion
attraction of water molecules to each other via hydrogen bonding
adhesion
attraction between water molecules and other molecules
surface tension
capacity of a substance to withstand rupturing when placed under tension or stress
capillary action
the movement of water up thin tubes made up of polar molecules
Figure Descriptions
Figure 8.2.1. The image shows a close-up view of a needle floating on the surface of water inside a glass measuring cup. The needle is metallic and elongated, positioned diagonally across the image. The water creates a visible meniscus where it meets the needle, indicating surface tension at work. In the background, there are red measurement markings on the glass, slightly out of focus, which curve around the inner surface of the measuring cup. The overall color scheme is muted, with shades of grey and silver dominating the scene. [Return to Figure 8.2.1]
Figure 8.2.2. The image depicts a simplified diagram illustrating capillary action. It shows a transparent cylindrical container, partially filled with a light blue liquid, labeled “Water.” In the center of the container, there is a narrow capillary tube that extends both into the liquid and above the level of the liquid surface. The liquid is shown rising up the tube due to capillary attraction, surpassing the liquid level in the container. Arrows point to the tube and the area of liquid rise, labeled “Capillary tube” and “Capillary attraction,” respectively. [Return to Figure 8.2.2]
Figure 8.2.3. The image shows a water strider insect gracefully standing on the surface of water. Its long, slender legs spread outwards, creating small dimples where they contact the water, demonstrating surface tension. The water is clear, revealing a bed of round, smooth pebbles below. The background is slightly blurred, giving a sense of depth and focus on the water strider at the center of the image. The insect’s body is dark brown and contrasts subtly against the reflective water surface. [Return to Figure 8.2.3]
Licenses and Attributions
“8.2 Water’s Cohesive and Adhesive Properties” is adapted from “2.2 Water” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “8.2 Water’s Cohesive and Adhesive Properties” is licensed under CC-BY-NC 4.0.
Media Attributions
- 1A.B.3 Water Surface Tension © Cory Zanker is licensed under a CC BY (Attribution) license
- 1A.B.3 Water Adhesive Force © Pearson-Scott Foresman, donated to the Wikimedia Foundation) is licensed under a CC BY (Attribution) license
- 1A.B.3 Water adhesion and cohesion © Tim Vickers is licensed under a CC BY (Attribution) license
attraction of water molecules to each other via hydrogen bonding
capacity of a substance such as water to withstand rupturing when placed under tension or stress
attraction between water molecules and other molecules
the movement of water up thin tubes made up of polar molecules
