2.1 Atoms, Ions, and Molecules
Learning Objectives
By the end of this section, you will be able to do the following:
- Define elements.
- Compare the ways in which electrons can be donated or shared between atoms.
- Explain the ways in which naturally occurring elements combine to create molecules.
Elements in various combinations comprise all matter, including living things. Some of the most abundant elements in living organisms include carbon, hydrogen, nitrogen, oxygen, sulfur, and phosphorus. These form the nucleic acids, proteins, carbohydrates, and lipids that are the fundamental components of living matter. Biologists must understand these important building blocks and the unique structures of the atoms that comprise molecules, allowing for cells, tissues, organ systems, and entire organisms to form.
All biological processes follow the laws of physics and chemistry, so in order to understand how biological systems work, it is important to understand the underlying physics and chemistry. For example, the flow of blood within the circulatory system follows the laws of physics that regulate the modes of fluid flow. The breakdown of the large, complex molecules of food into smaller molecules—and the conversion of these to release energy to be stored in adenosine triphosphate (ATP)—is a series of chemical reactions that follow chemical laws. The properties of water and the formation of hydrogen bonds are key to understanding living processes. Recognizing the properties of acids and bases is important, for example, to our understanding of the digestive process. Therefore, the fundamentals of physics and chemistry are important for gaining insight into biological processes.
Each element is designated by its chemical symbol, which is a single capital letter or, when the first letter is already “taken” by another element, a combination of two letters. Some elements follow the English term for the element, such as C for carbon and Ca for calcium. Other elements’ chemical symbols derive from their Latin names. For example, the symbol for sodium is Na, referring to natrium, the Latin word for sodium.
The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N). In the nonliving world, elements are found in different proportions, and some elements common to living organisms are radenosine triphosphate (ATP)elatively rare on the earth as a whole, as Table 2.1.1 shows. For example, the atmosphere is rich in nitrogen and oxygen but contains little carbon and hydrogen, while the earth’s crust, although it contains oxygen and a small amount of hydrogen, has little nitrogen and carbon. In spite of their differences in abundance, all elements and the chemical reactions between them obey the same chemical and physical laws regardless of whether they are a part of the living or nonliving world.
| Element | Life (Humans) | Atmosphere | Earth’s Crust |
|---|---|---|---|
| Oxygen (O) | 65% | 21% | 46% |
| Carbon (C) | 18% | trace | trace |
| Hydrogen (H) | 10% | trace | 0.1% |
| Nitrogen (N) | 3% | 78% | trace |
The Structure of the Atom
To understand how elements come together, we must first discuss the element’s smallest component or building block, the atom. An atom is the smallest unit of matter that retains all of the element’s chemical properties. For example, one gold atom has all of the properties of gold, like its chemical reactivity. A gold coin is simply a very large number of gold atoms molded into the shape of a coin and contains small amounts of other elements known as impurities. We cannot break down gold atoms into anything smaller while still retaining the properties of gold.
An atom is composed of two regions. The nucleus is in the atom’s center and contains protons and neutrons. The atom’s outermost region holds its electrons in orbit around the nucleus. Atoms contain protons, electrons, and neutrons, among other subatomic particles. The most common isotope of hydrogen (H) is the only exception and is made of one proton and one electron with no neutrons.
Chemical Reactions and Molecules
All elements are most stable when their outermost shell is filled with electrons according to the octet rule. This is because it is energetically favorable for atoms to be in that configuration and it makes them stable. However, since not all elements have enough electrons to fill their outermost shells, atoms form chemical bonds with other atoms thereby obtaining the electrons they need to attain a stable electron configuration.
An atom may give, take, or share electrons with another atom to achieve a full valence shell, the most stable electron configuration. When two or more atoms chemically bond with each other, the resultant chemical structure is a molecule. The familiar water molecule, H2O, consists of two hydrogen atoms and one oxygen atom. These bond together to form water, as Figure 2.1.2 illustrates. Atoms can form molecules by donating, accepting, or sharing electrons to fill their outer shells.
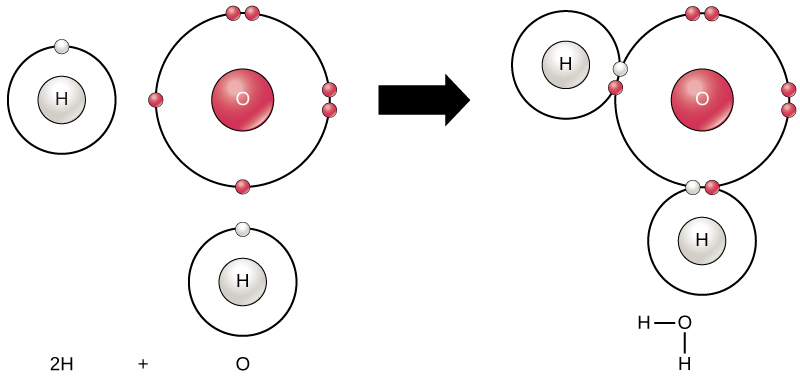
Chemical reactions occur when two or more atoms bond together to form molecules or when bonded atoms break apart. Scientists call the substances used in the beginning of a chemical reaction reactants (usually on the left side of a chemical equation), and we call the substances at the end of the reaction products (usually on the right side of a chemical equation). We typically draw an arrow between the reactants and products to indicate the chemical reaction’s direction. This direction is not always a “one-way street.” To create the water molecule above, the chemical equation would be:
2H + O → H2O
An example of a simple chemical reaction is breaking down hydrogen peroxide molecules, each of which consists of two hydrogen atoms bonded to two oxygen atoms (H2O2). The reactant hydrogen peroxide breaks down into water, containing one oxygen atom bound to two hydrogen atoms (H2O), and oxygen, which consists of two bonded oxygen atoms (O2). In the equation below, the reaction includes two hydrogen peroxide molecules and two water molecules. This is an example of a balanced chemical equation, wherein each element’s number of atoms is the same on each side of the equation. According to the law of conservation of matter, the number of atoms before and after a chemical reaction should be equal, such that no atoms are, under normal circumstances, created or destroyed.
2H2O2 (hydrogen peroxide) → 2H2O (water) + O2 (oxygen)
Even though all of the reactants and products of this reaction are molecules (each atom remains bonded to at least one other atom), in this reaction only hydrogen peroxide and water are representatives of compounds: They contain atoms of more than one type of element. Molecular oxygen, alternatively, as Figure 2.1.3 shows, consists of two doubly bonded oxygen atoms.
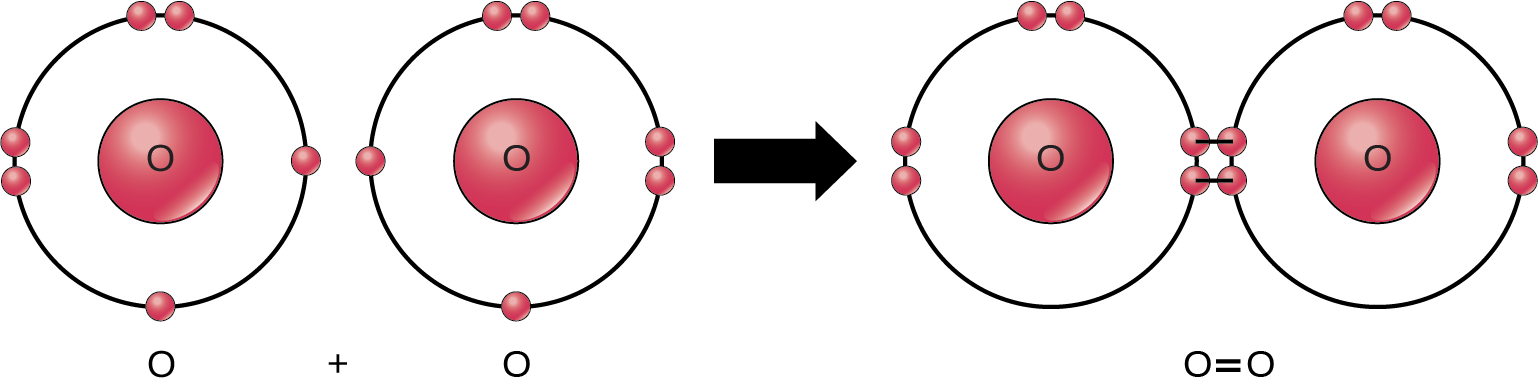
Some chemical reactions, such as the one above, can proceed in one direction until they expend all the reactants. The equations that describe these reactions contain a unidirectional arrow and are irreversible. Reversible reactions are those that can go in either direction. In reversible reactions, reactants turn into products, but when the product’s concentration goes beyond a certain threshold (characteristic of the particular reaction), some of these products convert back into reactants. At this point, product and reactant designations reverse. This back and forth continues until a certain relative balance between reactants and products occurs—a state called equilibrium. A chemical equation with a double headed arrow pointing towards both the reactants and products often denote these reversible reaction situations.For example, in human blood, excess hydrogen ions (H+) bind to bicarbonate ions (HCO3–) forming an equilibrium state with carbonic acid (H2CO3). If we added carbonic acid to this system, some of it would convert to bicarbonate and hydrogen ions.
HCO3−+ H+ ↔ H2CO3
However, biological reactions rarely obtain equilibrium because the concentrations of the reactants or products or both are constantly changing, often with one reaction’s product a reactant for another. To return to the example of excess hydrogen ions in the blood, forming carbonic acid will be the reaction’s major direction. However, the carbonic acid can also leave the body as carbon dioxide gas (via exhalation) instead of converting back to bicarbonate ion, thus driving the reaction to the right by the law of mass action. These reactions are important for maintaining homeostasis in our blood.
HCO3− + H+ ↔ H2CO3 ↔ CO2 + H2O
Ions and Ionic Bonds
Some atoms are more stable when they gain or lose an electron (or possibly two) and form ions. This fills their outermost electron shell and makes them energetically more stable. Because the number of electrons does not equal the number of protons, each ion has a net charge. Cations are positive ions that form by losing electrons. Negative ions form by gaining electrons, which we call anions. We designate anions by their elemental name and change the ending to “-ide,” thus the anion of chlorine is chloride, and the anion of sulfur is sulfide.
Scientists refer to this movement of electrons from one element to another as electron transfer. As Figure 2.1.4 illustrates, sodium (Na) only has one electron in its outer electron shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. We now refer to it as a sodium ion. Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. We now refer to it as a chloride ion. In this example, sodium will donate its one electron to empty its shell, and chlorine will accept that electron to fill its shell. Both ions now satisfy the octet rule and have complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium cation) or –1 (chloride anion) charge. Note that these transactions can normally only take place simultaneously: in order for a sodium atom to lose an electron, it must be in the presence of a suitable recipient like a chlorine atom.
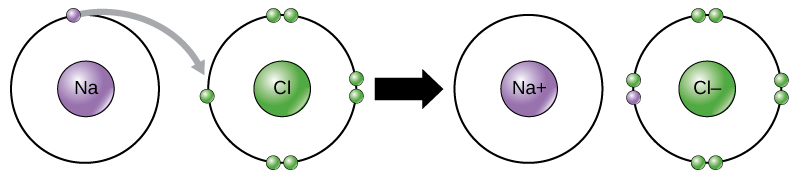
Ionic bonds form between ions with opposite charges. For instance, positively charged sodium ions and negatively charged chloride ions bond together to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge.Physiologists refer to certain salts as electrolytes (including sodium, potassium, and calcium), ions necessary for nerve impulse conduction, muscle contractions, and water balance. Many sports drinks and dietary supplements provide these ions to replace those lost from the body via sweating during exercise.
Covalent Bonds
Another way to satisfy the octet rule is by sharing electrons between atoms to form covalent bonds. These bonds are stronger and much more common than ionic bonds in the molecules of living organisms. We commonly find covalent bonds in carbon-based organic molecules, such as our DNA and proteins. We also find covalent bonds in inorganic molecules like H2O, CO2, and O2. The bonds may share one, two, or three pairs of electrons, making single, double, and triple bonds, respectively. The more covalent bonds between two atoms, the stronger their connection. Thus, triple bonds are the strongest.
The strength of different levels of covalent bonding is one of the main reasons living organisms have a difficult time in acquiring nitrogen for use in constructing their molecules, even though molecular nitrogen, N2, is the most abundant gas in the atmosphere. Molecular nitrogen consists of two nitrogen atoms triple bonded to each other and, as with all molecules, sharing these three pairs of electrons between the two nitrogen atoms allows for filling their outer electron shells, making the molecule more stable than the individual nitrogen atoms. This strong triple bond makes it difficult for living systems to break apart this nitrogen in order to use it as constituents of proteins and DNA.
Forming water molecules provides an example of covalent bonding. Covalent bonds bind the hydrogen and oxygen atoms that combine to form water molecules, as Figure 2.1.2 shows. The electron from the hydrogen splits its time between the hydrogen atoms’ incomplete outer shell and the oxygen atoms’ incomplete outer shell. To completely fill the oxygen’s outer shell, which has six electrons but which would be more stable with eight, two electrons (one from each hydrogen atom) are needed: hence, the well-known formula H2O. The two elements share the electrons to fill the outer shell of each, making both elements more stable.
Covalent bonds come in two flavors: polar covalent and nonpolar covalent bonds. We will explore the differences between these subtypes and how you can identify them in the next section.
Practice Questions
Glossary
matter
physical substance that which occupies space and possesses mass.
element
form of matter with specific chemical and physical properties that cannot break down into smaller substances by ordinary chemical reactions. The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N).
molecule
ion
ionic bond
form between ions with opposite charges.
covalent bond
a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
Figure Descriptions
Figure 2.1.1. The image depicts a 3D molecular model of dopamine against a solid blue background. The structure consists of a series of interconnected spheres of various colors, representing different atoms. Six grey spheres form a hexagonal ring at the center, with two red spheres attached to the ring, indicating the presence of oxygen atoms. Additional grey, white, and one blue sphere are connected in a branching pattern away from the hexagonal ring. The white spheres represent hydrogen atoms, grey spheres represent carbon atoms, and the single blue sphere represents a nitrogen atom. The spheres are connected by dark grey rods, illustrating the chemical bonds between the atoms. [Return to Figure 2.1.1]
Figure 2.1.2. The image depicts the chemical formation of a water molecule (H2O) from hydrogen and oxygen atoms. On the left, there are two separate hydrogen (H) atoms and one oxygen (O) atom. Each hydrogen atom is illustrated with a large white circle containing a smaller “H” at the center, and one small red circle (electron) orbiting around it. The oxygen atom is shown with a large red circle containing a smaller “O” at the center, surrounded by six small red circles (electrons) arranged in two orbits. To the right, an arrow points to a water molecule consisting of one oxygen atom bonded to two hydrogen atoms. The water molecule is depicted with the oxygen atom at the center with two hydrogen atoms connected by white lines, representing bonds. [Return to Figure 2.1.2]
Figure 2.1.3. The image illustrates the formation of an oxygen molecule (O₂) through a covalent bond. It consists of three primary sections. On the left, there are two separate depictions of oxygen atoms. Each oxygen atom has a central nucleus represented by a red circle with the letter “O” inside it. Surrounding each nucleus are two concentric circles representing electron shells. The inner shell has two electrons while the outer shell has six electrons depicted as smaller red circles. In the center, a large black arrow points to the right, indicating that the reaction proceeds from left to right. On the right, two oxygen atoms are shown sharing electrons, forming a double bond. The illustration depicts the two central oxygen atoms closer together, with the shared pairs of electrons between them highlighted to signify the double bond. The shells now overlap to show the sharing of electrons. [Return to Figure 2.1.3]
Figure 2.1.4. The image illustrates the ionic bonding process between a sodium (Na) atom and a chlorine (Cl) atom. On the left side, a purple circle labeled “Na” is surrounded by a single smaller purple dot in its outer ring. Adjacent to it is a green circle labeled “Cl” with seven smaller green dots in its outer ring. There is a grey arrow pointing from the sodium atom’s outermost electron (small purple dot) to the chlorine atom’s outer ring, signifying the transfer of one electron. In the center of the image, there is a large black arrow pointing rightwards, indicating the progression from left to right. On the right side of the image, there is a purple circle labeled “Na+” without any smaller dots in its outer ring, indicating it has lost an electron and is now a positively charged ion. Next to it is a green circle labeled “Cl-” with eight smaller green dots, including the newly added purple dot, in its outer ring, indicating it has gained an electron and is now a negatively charged ion. [Return to Figure 2.1.4]
Licenses and Attributions
“Atoms, Ions, and Molecules” is adapted from “2.1 Atoms, Isotopes, Ions and Molecules: The Building Blocks” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “Atoms, Ions, and Molecules” is licensed under CC-BY-NC 4.0.
Media Attributions
- Dopamine_finalver © Panisanum is licensed under a CC BY-SA (Attribution ShareAlike) license
- 1A.B.2 Water bonding © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- Oxygen bonding © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- Ionic bonds NaCl © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
form of matter with specific chemical and physical properties that cannot break down into smaller substances by ordinary chemical reactions. The four elements common to all living organisms are oxygen (O), carbon (C), hydrogen (H), and nitrogen (N).
physical substance that which occupies space and possesses mass.
a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
high energy molecule used as currency for cellular processes
atoms or molecules with a net electric charge due to the loss or gain of one or more electrons.
form between ions with opposite charges.
chemical bonds that involve the sharing of electrons to form electron pairs between atoms.
