2.4 Carbon
Learning Objectives
By the end of this section, you will be able to do the following:
- Explain why carbon is important for life.
- Identify the properties of common functional groups in biological molecules.
Many complex molecules called macromolecules, such as proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids, comprise cells. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
Hydrocarbons
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4) described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which releases when these molecules burn (oxidize). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms.
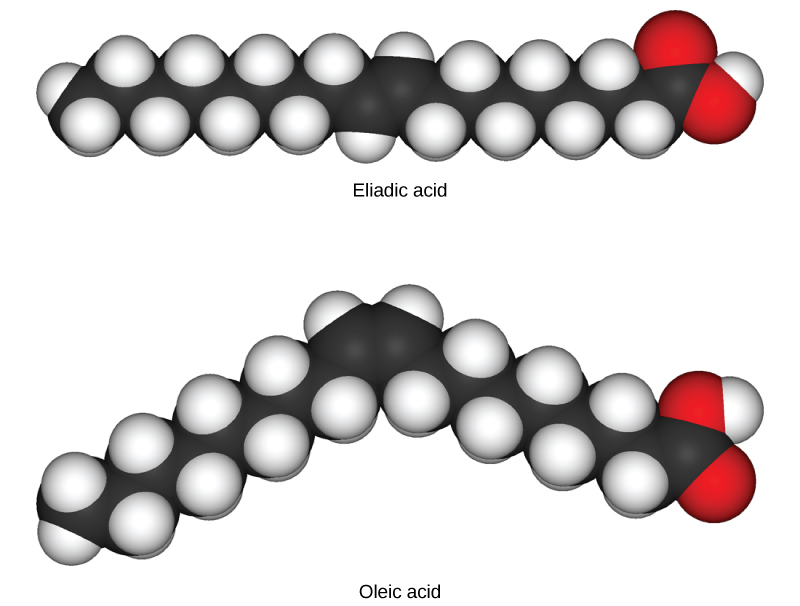
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds, and each type of bond affects the molecule’s geometry in a specific way. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.
Isomers
The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. We call molecules that share the same chemical formula but differ in the placement (structure) of their atoms and/or chemical bonds isomers.
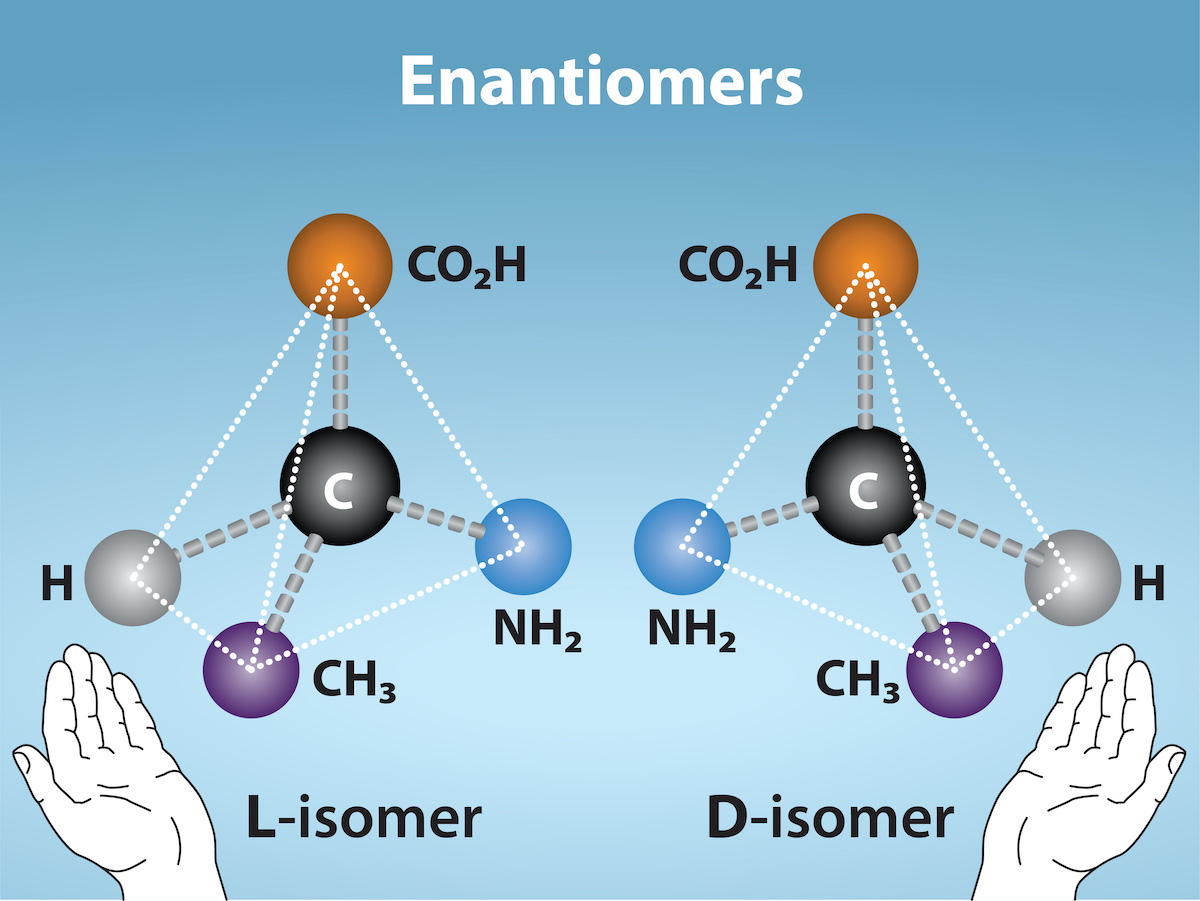
Enantiomers
Enantiomers are molecules that share the same chemical structure and chemical bonds but differ in the three-dimensional placement of atoms so that they are non-superimposable mirror images. Figure 2.4.2 shows an amino acid alanine example, where the two structures are nonsuperimposable. In nature, the L-forms of amino acids are predominant in proteins. Some D forms of amino acids are seen in the cell walls of bacteria and polypeptides in other organisms. Similarly, the D-form of glucose is the main product of photosynthesis and we rarely see the molecule’s L-form in nature.
Functional Groups
Functional groups are groups of atoms that occur within molecules and confer specific chemical properties to those molecules. We find them along the “carbon backbone” of macromolecules. Chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen form this carbon backbone. Molecules with other elements in their carbon backbone are substituted hydrocarbons.
The functional groups in a macromolecule are usually attached to the carbon backbone at one or several different places along its chain and/or ring structure. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
A functional group can participate in specific chemical reactions. Important functional groups in biological molecules include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl. These groups play an important role in forming molecules like DNA, proteins, carbohydrates, and lipids. We usually classify functional groups as hydrophobic or hydrophilic depending on their charge or polarity characteristics. An example of a hydrophobic group is the nonpolar methyl molecule. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
Among the hydrophilic functional groups is the carboxyl group in amino acids, some amino acid side chains, and the fatty acids that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H+) from the COOH group resulting in the negatively charged COO- group. This contributes to the hydrophilic nature of whatever molecule on which it is found. Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate.
Practice Questions
Glossary
hydrocarbons
organic molecules consisting entirely of carbon and hydrogen
functional groups
groups of atoms that occur within molecules and confer specific chemical properties to those molecules.
macromolecule
large molecules made up of repeating units found in living organisms. These include DNA, proteins, carbohydrates, and lipids.
Figure Descriptions
Figure 2.4.1. The image displays two molecular structures labeled as “Elaidic acid” and “Oleic acid.” Both structures are depicted using a ball-and-stick model, where spheres represent atoms, and sticks denote the bonds between them. The top structure, Elaidic acid, appears as a straight chain composed mainly of white and dark grey spheres. The white spheres signify hydrogen atoms, and the dark grey spheres indicate carbon atoms. At the right end, there are two bright red spheres representing oxygen atoms. The bottom structure, Oleic acid, is similar but features a distinct bend in its chain, with similar colors for hydrogen, carbon, and oxygen atoms. Below each molecular structure, the respective chemical name is written. [Return to Figure 2.4.1]
Figure 2.4.2. The image is a diagram illustrating the concept of enantiomers, depicting two mirror-image molecules labeled as “L-isomer” and “D-isomer.” Each molecule contains four distinct groups bonded to a central black sphere labeled “C,” representing a carbon atom. These groups are colored and include: a grey sphere labeled “H” (hydrogen), an orange sphere labeled “CO₂H” (carboxyl group), a purple sphere labeled “CH₃” (methyl group), and a blue sphere labeled “NH₂” (amine group). The bonds connecting these groups to the carbon are depicted as rods, with some appearing solid and others dashed, indicating different spatial orientations. Below each molecule, a graphic of a hand is shown with the palm facing toward the molecule, symbolizing the mirror relationship. The background is a gradient of light blue. At the top, the term “Enantiomers” is written in large white text. [Return to Figure 2.4.2]
Licenses and Attributions
“2.4 Carbon” is adapted from “2.3 Carbon” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “2.4 Carbon” is licensed under CC-BY-NC 4.0.
Media Attributions
- 1A.B Fats © Clark, Douglas, and Choi is licensed under a CC BY (Attribution) license
- 1A.B-Enantiomers © A. Rao, A. Hawkins, S. Fletcher and K. Ryan for Department of Biology, Texas A&M University is licensed under a CC BY (Attribution) license
organic molecules consisting entirely of carbon and hydrogen
groups of atoms that occur within molecules and confer specific chemical properties to those molecules.
large molecules made up of repeating units found in living organisms. These include DNA,, proteins, carbohydrates, and lipids.
