11.4 Heat Conservation and Dissipation
Malia Hoey and Christelle Sabatier
Learning Objectives
By the end of this section, you will be able to do the following:
- Describe how animals conserve and dissipate heat through structural, physiological, and behavioral adaptations.
- Explain how the thermal properties of water contribute to temperature regulation in organisms and environments.
Properties of Water
Heat Capacity
Water’s high heat capacity is a property that hydrogen bonding among water molecules causes. Water has the highest specific heat capacity of any liquid. We define specific heat as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie. It therefore takes water a long time to heat and a long time to cool. In fact, water’s specific heat capacity is about five times more than that of sand. This explains why the land cools faster than the sea. Due to its high heat capacity, warm blooded animals use water to more evenly disperse heat in their bodies: it acts in a similar manner to a car’s cooling system, transporting heat from warm places to cool places, causing the body to maintain a more even temperature.
Heat of Vaporization
Water also has a high heat of vaporization, the amount of energy required to change one gram of a liquid substance to a gas. A considerable amount of heat energy (586 cal) is required to accomplish this change in water. This process occurs on the water’s surface. As liquid water heats up, hydrogen bonding makes it difficult to separate the liquid water molecules from each other, which is required for it to enter its gaseous phase (steam). As a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain alcohol), whose hydrogen bonding with other ethanol molecules is weaker than water’s hydrogen bonding. Eventually, as water reaches its boiling point of 100°C (212°F), the heat is able to break the hydrogen bonds between the water molecules, and the kinetic energy (motion) between the water molecules allows them to escape from the liquid as a gas. Even when below its boiling point, water’s individual molecules acquire enough energy from other water molecules such that some surface water molecules can escape and vaporize; we call this process evaporation.
The fact that hydrogen bonds need to be broken for water to evaporate means that bonds use a substantial amount of energy in the process. As the water evaporates, energy is taken up by the process, cooling the environment where the evaporation is taking place. In many living organisms, including in humans, the evaporation of sweat, which is 90% water, allows the organism to cool so that it can maintain homeostasis of body temperature.
Basic Mechanisms of Heat Transfer
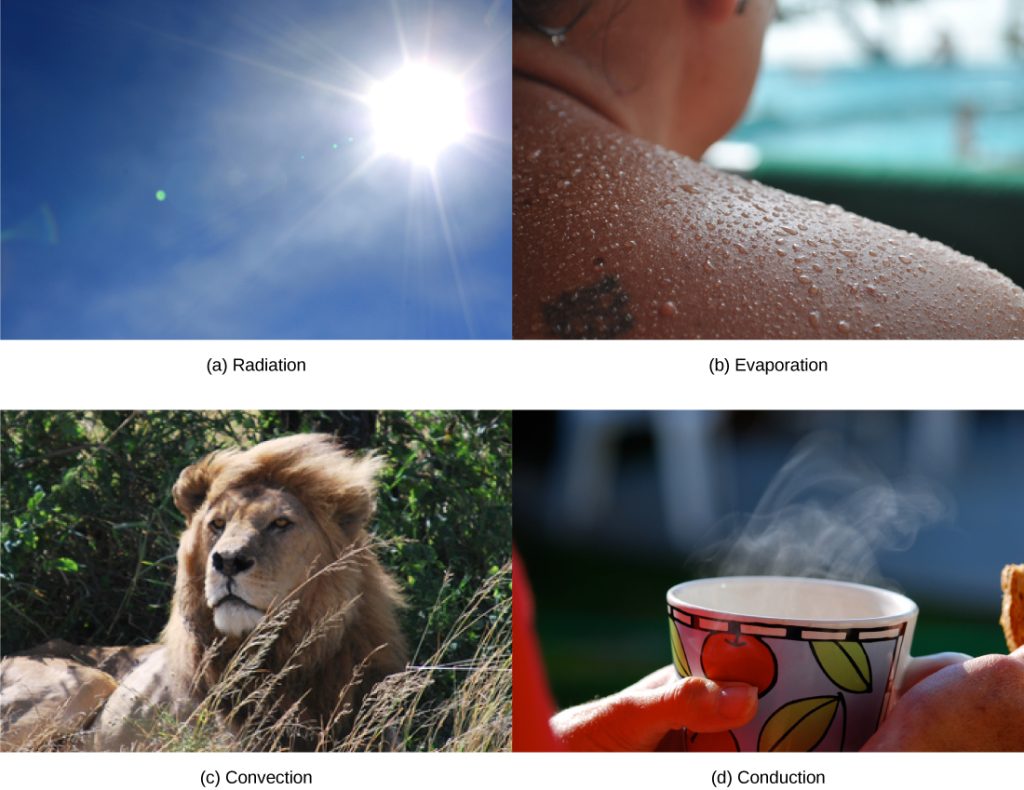
Heat can be exchanged between an animal and its environment through four mechanisms: radiation, evaporation, convection, and conduction (Figure 11.4.1). Radiation is the emission of electromagnetic “heat” waves. Heat comes from the sun in this manner and radiates from dry skin the same way. Heat can be removed with liquid from a surface during evaporation. This occurs when a mammal sweats. Convection currents of air remove heat from the surface of dry skin as the air passes over it. Heat will be conducted from one surface to another during direct contact with the surfaces, such as an animal resting on a warm rock.
Heat Conservation and Dissipation Strategies
Animals conserve or dissipate heat in a variety of ways. Endothermic animals often rely on insulation to retain warmth, especially in cold climates. This insulation can present in the form of fur, feathers, fat, or a combination of these adaptations. For example, animals with thick fur or feathers trap a layer of air close to the skin, creating an insulating barrier that slows heat loss to the environment. Polar bears and seals, despite living and swimming in subfreezing conditions, maintain a consistently warm internal body temperature through such insulation. The arctic fox, another cold-adapted mammal, curls up and wraps its fluffy tail around its body while sleeping, adding an extra layer of warmth.
Many mammals also exhibit piloerection, commonly known as “goose bumps,” in which small muscles (arrector pili) contract to make hairs stand on end. This traps a thicker layer of insulating air near the skin, especially useful when the body generates additional heat through shivering. In addition to hair and feathers, fat, particularly subcutaneous fat or blubber, serves as an effective insulator. However, if an animal loses a significant amount of body fat, its ability to conserve heat may be compromised, increasing vulnerability to cold environments.
Endothermic animals use their circulatory systems to help regulate body temperature. Two primary mechanisms are vasodilation and vasoconstriction. Vasodilation increases blood flow to the skin’s surface. This brings heat from the body’s core closer to the surface, promoting radiative and evaporative heat loss, and thereby cooling the body. Vasoconstriction reduces blood flow to the skin by narrowing peripheral blood vessels. This redirects blood toward the body’s core, helping to conserve heat and protect vital organs.
Some animals have specialized circulatory adaptations such as countercurrent heat exchange. In this system, warm arterial blood flowing from the body’s core transfers heat to cooler venous blood returning from the periphery. This warms the returning blood and prevents cold blood from cooling down into internal organs like the heart. Countercurrent heat exchange can also be temporarily reduced or shut down in some species to prevent overheating. This adaptation is found in a variety of species, including dolphins, sharks, bony fish, bees, and hummingbirds.
In contrast, certain animals have evolved vascular adaptations that enhance heat loss. For example, dolphin flukes and elephant ears contain dense networks of blood vessels that increase surface area for heat dissipation. When blood flow to these structures increases, heat is lost more efficiently to the environment, helping to cool the animal when needed.

Water itself plays a vital role in thermoregulation due to its unique physical properties. Water has a high specific heat capacity, meaning it can absorb or release large amounts of heat with little change in temperature. This helps stabilize both internal body temperatures and external aquatic environments, providing thermal buffering for aquatic animals. Water also has a high heat of vaporization, making evaporation an efficient way to cool the body. For example, sweating in mammals or evaporative cooling in reptiles uses water loss to remove excess heat. These properties make water essential to both active and passive cooling strategies. In addition, water’s unique property of becoming less dense as ice means it floats, forming insulating layers on the surface of aquatic habitats. This phenomenon protects the liquid water below from freezing solid, thereby safeguarding aquatic organisms from lethal cold temperatures
Ground squirrels offer a compelling example of how animals adjust their activities to regulate temperature and maintain thermal balance. During the colder months, many species are known for their remarkable ability to enter hibernation, a prolonged state of dormancy where their metabolic rate can plummet by up to 98%, drastically reducing internal heat production and conserving vital energy reserves. To avoid the intense heat of the summer months, ground squirrels may burrow deeper into the ground, seeking the cooler and more stable subterranean temperatures which allow for effective heat dissipation through conduction to the soil, minimizing heat gain from solar radiation and convection at the surface. Furthermore, ground squirrels exhibit torpor, a state of significantly reduced physiological activity characterized by a dramatic drop in temperature. These shorter bouts of torpor, which can occur daily or seasonally, allow them to conserve energy and reduce heat loss rapidly in response to acute cold conditions or periods of food scarcity.
Some ectothermic animals use changes in their behavior to help regulate body temperature. For example, a desert ectothermic animal may simply seek cooler areas during the hottest part of the day in the desert to keep from getting too warm. The same animals may climb onto rocks to capture heat during a cold desert night. Some animals seek water to aid evaporation in cooling them, as seen with reptiles. Other ectotherms use group activity such as the activity of bees to warm a hive to survive winter.
Many animals, especially mammals, use metabolic waste heat as a heat source. When muscles are contracted, most of the energy from the ATP used in muscle actions is wasted energy that translates into heat. Severe cold elicits a shivering reflex that generates heat for the body. Many species also have a type of adipose tissue called brown fat that specializes in generating heat.
Video 11.2. Hibernation in Arctic Ground Squirrels by PolarTREC
Practice Questions
Glossary
heat capacity
amount of heat one gram of substance needs to absorb or lose to change its temperature by one degree Celsius
heat of vaporization
amount of heat required to turn one gram of a liquid into a vapor without a rise in the temperature of the liquid
radiation
heat from electromagnetic infrared light such as comes from the sun
evaporation
transition from liquid to gas, which removes heat in the process
convection
heat removed by air from dry skin
conduction
heat transferred from one surface to another
vasodilation
blood vessels expand to increase blood flow to the body surface, encourages heat loss
vasoconstriction
blood vessels constrict to reduce blood flow to the skin and encourages heat conservation
Figure Descriptions
Figure 11.4.1. The image is a collage of four separate images arranged in two rows, each depicting a different method of heat transfer or temperature regulation. In the top left, labeled “(a) Radiation,” is a photograph of a clear blue sky with the sun shining brightly, indicating solar radiation. The top right, labeled “(b) Evaporation,” shows a close-up of a person’s wet shoulder with beads of water, suggesting the process of evaporation under sunlight. The bottom left, labeled “(c) Convection,” captures a lion resting in the grass, likely warming itself in the sun, illustrating the transfer of heat through air movement. The bottom right, labeled “(d) Conduction,” features a hand holding a colorful cup with steam rising from it, implying heat transfer from a hot liquid. [Return to Figure 11.4.1]
Figure 11.4.2. Photo of an antelope jackrabbit highlighting its well vascularized large ears which it uses to regulate its internal body temperature. [Return to Figure 11.4.2]
Licenses and Attributions
“11.4 Heat Conservation and Dissipation” is adapted from “33.3 Homeostasis” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e under CC-BY 4.0. “11.4 Heat Conservation and Dissipation” is licensed under CC-BY-NC 4.0.
Media Attributions
- 1A.B-Heat-Mechanisms © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- 1A.B-Lepus_alleni © Francisco Farriols Sarabia is licensed under a CC BY-NC (Attribution NonCommercial) license
The amount of heat one gram of substance needs to absorb or lose to change its temperature by one degree Celsius.
The amount of heat required to turn 1g of a liquid into a vapor without a rise in the temperature of the liquid
attractive force between partially positive and partially negative atoms that are part of polar covalent bonds in separate molecules.
heat from electromagnetic infrared light such as comes from the sun
transition from liquid to gas, which removes heat in the process
Heat removed by air from dry skin
Heat transferred from one surface to another
Storing body heat
Releasing body heat
blood vessels expand to increase blood flow to the body surface, encourages heat loss
blood vessels constrict to reduce blood flow to the skin and encourages heat conservation
