21.3 Insulin and Glucagon Signaling
Learning Objectives
By the end of this section, you will be able to do the following:
- Explain how G-protein coupled receptors work
- Explain how enzyme-linked receptors work
Hormones mediate changes in target cells by binding to specific hormone receptors. In this way, even though hormones circulate throughout the body and come into contact with many different cell types, they only affect cells that possess the necessary receptors. Receptors for a specific hormone may be found on many different cells or may be limited to a small number of specialized cells. For example, thyroid hormones act on many different tissue types, stimulating metabolic activity throughout the body. Cells can have many receptors for the same hormone but often also possess receptors for different types of hormones. The number of receptors that respond to a hormone determines the cell’s sensitivity to that hormone, and the resulting cellular response. Additionally, the number of receptors that respond to a hormone can change over time, resulting in increased or decreased cell sensitivity. In up-regulation, the number of receptors increases in response to rising hormone levels, making the cell more sensitive to the hormone and allowing for more cellular activity. When the number of receptors decreases in response to rising hormone levels, called down-regulation, cellular activity is reduced.
Receptor binding alters cellular activity and results in an increase or decrease in normal body processes. Depending on the location of the protein receptor on the target cell and the chemical structure of the hormone, hormones can mediate changes directly by binding to intracellular hormone receptors and modulating gene transcription, or indirectly by binding to cell surface receptors and stimulating signaling pathways.
Cell-Surface Receptors
Cell-surface receptors are involved in most of the signaling in multicellular organisms. There are three general categories of cell-surface receptors: ion channel-linked receptors, G-protein coupled receptors (GPCRs), and enzyme-linked receptors. We’ll focus on the latter two here and discuss ion channel-linked receptors in the next section.
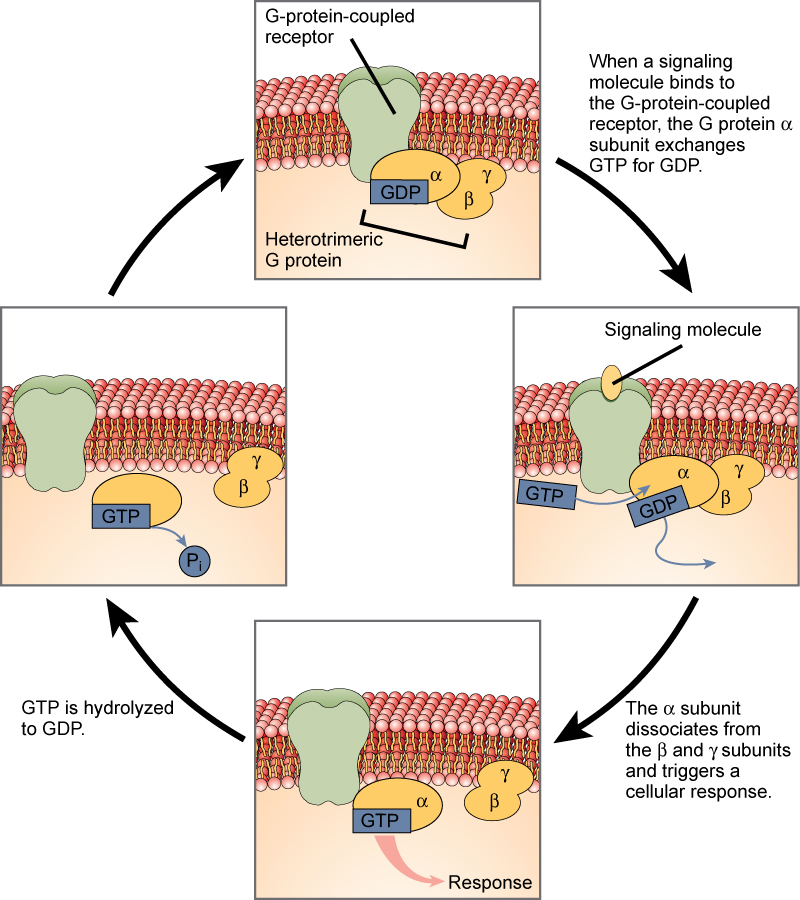
Glucagon Receptor – a G-protein coupled receptor (GPCR)
GPCRs bind a ligand and activate a membrane protein called a G-protein. The activated G-protein then interacts with either an ion channel or an enzyme in the membrane. All G-protein-linked receptors have seven transmembrane domains, but each receptor has its own specific extracellular domain and G-protein-binding site.
Cell signaling using G-protein-linked receptors occurs as a cyclic series of events. Before the ligand binds, the inactive G-protein can bind to a newly revealed site on the receptor specific for its binding. Once the G-protein binds to the receptor, the resultant shape change activates the G-protein, which releases GDP and picks up GTP. The subunits of the G-protein then split into the α subunit and the βγ subunit. One or both of these G-protein fragments may be able to activate other proteins as a result. After awhile, the GTP on the active α subunit of the G-protein is hydrolyzed to GDP and the βγ subunit is deactivated. The subunits reassociate to form the inactive G-protein and the cycle begins anew.
Insulin Receptor – a receptor tyrosine kinase (RTK)
Enzyme-linked receptors are cell-surface receptors with intracellular domains that are associated with an enzyme. In some cases, the intracellular domain of the receptor itself is an enzyme. Other enzyme-linked receptors have a small intracellular domain that interacts directly with an enzyme. The enzyme-linked receptors normally have large extracellular and intracellular domains, but the membrane-spanning region consists of a single alpha-helical region of the peptide strand. When a ligand binds to the extracellular domain, a signal is transferred through the membrane, activating the enzyme. Activation of the enzyme sets off a chain of events within the cell that eventually leads to a response. One example of this type of enzyme-linked receptor is the tyrosine kinase receptor (Figure 2). A kinase is an enzyme that transfers phosphate groups from ATP to another protein. The tyrosine kinase receptor transfers phosphate groups to tyrosine molecules (tyrosine residues). First, signaling molecules bind to the extracellular domain of two nearby tyrosine kinase receptors. The two neighboring receptors then bond together, or dimerize. Phosphates are then added to tyrosine residues on the intracellular domain of the receptors (phosphorylation). The phosphorylated residues can then transmit the signal to the next messenger within the cytoplasm.
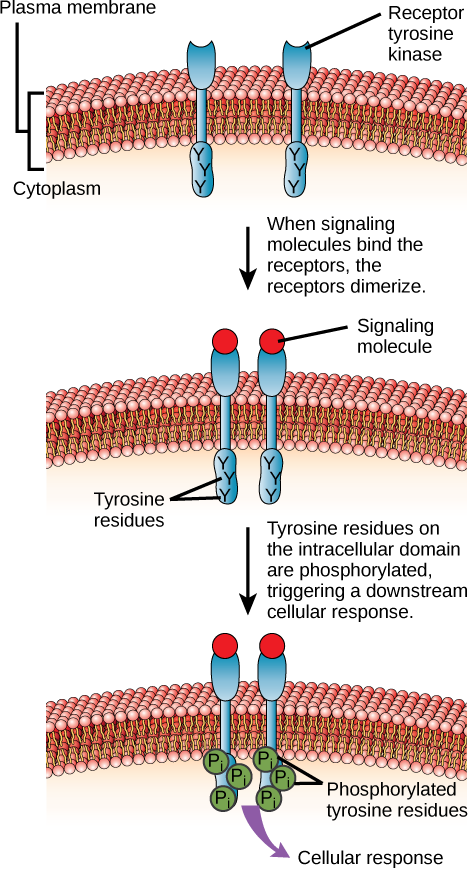
Figure Descriptions
Figure 1.
Figure 2.
