21.3 Insulin and Glucagon Signaling
Learning Objectives
By the end of this chapter, you will be able to do the following:
- Explain how G-protein-coupled receptors work.
- Explain how enzyme-linked receptors work.
Hormones mediate changes in target cells by binding to specific hormone receptors. In this way, even though hormones circulate throughout the body and come into contact with many different cell types, they only affect cells that possess the necessary receptors. Receptors for a specific hormone may be found on many different cells or may be limited to a small number of specialized cells. For example, thyroid hormones act on many different tissue types, stimulating metabolic activity throughout the body. Cells can have many receptors for the same hormone but often also possess receptors for different types of hormones. The number of receptors that respond to a hormone determines the cell’s sensitivity to that hormone, and the resulting cellular response. Additionally, the number of receptors that respond to a hormone can change over time, resulting in increased or decreased cell sensitivity. In up-regulation, the number of receptors increases in response to rising hormone levels, making the cell more sensitive to the hormone and allowing for more cellular activity. When the number of receptors decreases in response to rising hormone levels, called down-regulation, cellular activity is reduced.
Receptor binding alters cellular activity and results in an increase or decrease in normal body processes. Depending on the location of the protein receptor on the target cell and the chemical structure of the hormone, hormones can mediate changes directly by binding to intracellular hormone receptors and modulating gene transcription, or indirectly by binding to cell surface receptors and stimulating signaling pathways.
Cell-Surface Receptors
Cell-surface receptors are involved in most of the signaling in multicellular organisms. There are three general categories of cell-surface receptors: ion channel-linked receptors, G-protein-coupled receptors (GPCRs), and enzyme-linked receptors. We’ll focus on the latter two here and discuss ion channel-linked receptors in the next section.
Glucagon Receptor: A G-Protein-Coupled Receptor (GPCR)
GPCRs bind a ligand and activate a membrane protein called a G-protein. The activated G-protein then interacts with either an ion channel or an enzyme in the membrane. All G-protein-linked receptors have seven transmembrane domains, but each receptor has its own specific extracellular domain and G-protein-binding site.
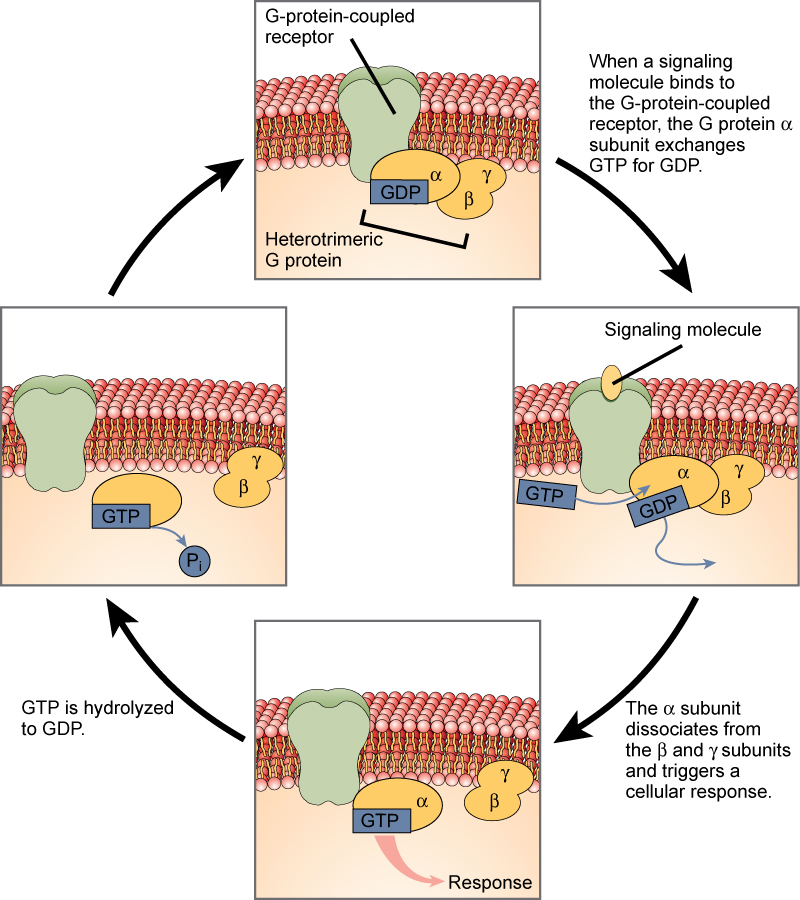
Cell signaling using G-protein-linked receptors occurs as a cyclic series of events (Figure 21.3.1). Before the ligand binds, the inactive G-protein can bind to a newly revealed site on the receptor specific for its binding. Once the G-protein binds to the receptor, the resultant shape change activates the G-protein, which releases GDP and picks up GTP. The subunits of the G-protein then split into the α subunit and the βγ subunit. One or both of these G-protein fragments may be able to activate other proteins as a result. After awhile, the GTP on the active α subunit of the G-protein is hydrolyzed to GDP and the βγ subunit is deactivated. The subunits reassociate to form the inactive G-protein and the cycle begins anew.
Heterotrimeric G proteins have three subunits: α, β, and γ. When a signaling molecule binds to a G-protein-coupled receptor in the plasma membrane, a GDP molecule associated with the α subunit is exchanged for GTP. The β and γ subunits dissociate from the α subunit, and a cellular response is triggered either by the α subunit or the dissociated βγ pair. Hydrolysis of GTP to GDP terminates the signal.
Insulin Receptor: A Receptor Tyrosine Kinase (RTK)
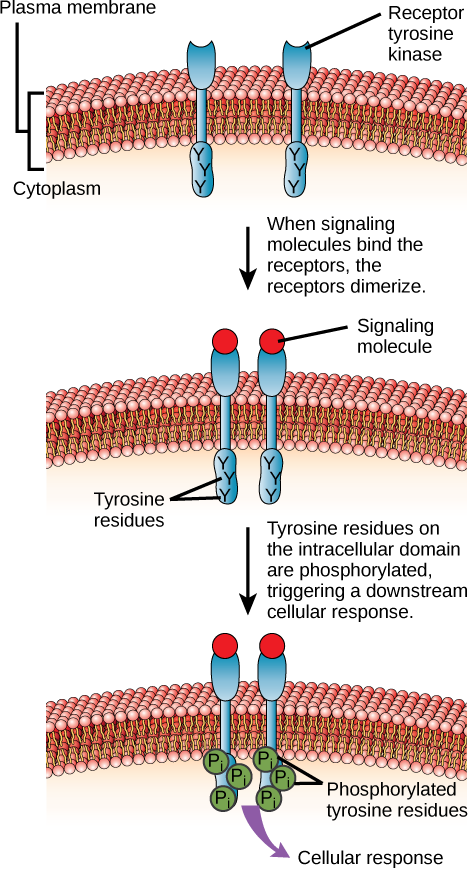
Enzyme-linked receptors are cell-surface receptors with intracellular domains that are associated with an enzyme. In some cases, the intracellular domain of the receptor itself is an enzyme. Other enzyme-linked receptors have a small intracellular domain that interacts directly with an enzyme. The enzyme-linked receptors normally have large extracellular and intracellular domains, but the membrane-spanning region consists of a single alpha-helical region of the peptide strand. When a ligand binds to the extracellular domain, a signal is transferred through the membrane, activating the enzyme. Activation of the enzyme sets off a chain of events within the cell that eventually leads to a response. One example of this type of enzyme-linked receptor is the tyrosine kinase receptor (Figure 21.3.2). A kinase is an enzyme that transfers phosphate groups from ATP to another protein. The tyrosine kinase receptor transfers phosphate groups to tyrosine molecules (tyrosine residues). First, signaling molecules bind to the extracellular domain of two nearby tyrosine kinase receptors. The two neighboring receptors then bond together, or dimerize. Phosphates are then added to tyrosine residues on the intracellular domain of the receptors (phosphorylation). The phosphorylated residues can then transmit the signal to the next messenger within the cytoplasm.
A receptor tyrosine kinase is an enzyme-linked receptor with a single transmembrane region, and extracellular and intracellular domains. Binding of a signaling molecule to the extracellular domain causes the receptor to dimerize. Tyrosine residues on the intracellular domain are then autophosphorylated, triggering a downstream cellular response. The signal is terminated by a phosphatase that removes the phosphates from the phosphotyrosine residues.
Practice Questions
Glossary
insulin receptor
Receptor tyrosine kinase that binds to the ligand insulin and stimulates a response in insulin sensitive cells.
glucagon receptor
G protein coupled receptor that binds to the ligand glucagon and stimulates a response in glucagon sensitive cells.
heterotrimeric G protein
Three proteins named alpha, beta and gamma that in their inactive state bind to each other and a G protein coupled receptor (GPCR). G alpha binds GDP when inactive and is stimulated to bind GTP when activated by ligand binding to the GPCR. This releases G beta and gamma to interact with downstream protein effectors in the cell.
kinase
Enzyme that phosphorylates other proteins by adding a phosphate group to either serine, threonine or tyrosine residues.
Figure Descriptions
Figure 21.3.1. The image is a diagram illustrating the G-protein-coupled receptor (GPCR) signaling process. The process is shown in a cycle with several stages, each depicted in a separate part of the diagram. In the top section, a G-protein-coupled receptor (GPCR) is embedded in a cell membrane, with a G-protein complex. The complex includes a GDP molecule bound to an alpha subunit and the beta and gamma subunits nearby (labeled Heterotrimeric G protein). This configuration indicates an inactive state. An arrow points from this top section the section on the right with the text transcribed: When a signaling molecule binds to the G-protein-coupled receptor, the G protein alpha subunit exchanges GTP for GDP. To the right, a signaling molecule binds to the receptor, causing a conformational change. This results in the G protein alpha subunit exchanging GTP for GDP. The bottom right section shows the alpha subunit activating by dissociating from the beta and gamma subunits, triggering a cellular response. An arrow points to the bottom left section with the transcribed text: GTP is hydrolyzed to GDP. In the bottom left section, GTP bound to the alpha subunit is hydrolyzed back to GDP. The cycle completes as the alpha subunit returns to its original state bound to GDP. Connecting arrows indicate the sequential steps of the signaling pathway. [Return to Figure 21.3.1]
Figure 21.3.2. The image illustrates a series of steps depicting receptor tyrosine kinase (RTK) activation and signal transduction across a cell membrane. The first panel shows two inactive, separate RTK proteins (labeled “Receptor tyrosine kinase”) embedded in a lipid bilayer (labeled “cytoplasm”), colored pink and yellow, representing the cell membrane (labeled “plasma membrane”). The proteins are depicted with blue extracellular domains and dark blue intracellular domains with three “Y” figures in each RTK. An arrow points from the first panel to the second panel with the transcribed text: When the signaling molecules bind the receptors, the receptors dimerize. The second panel shows the receptors dimerized, with two red signaling molecules bound to the extracellular domain (labeled “Tyrosine residues”), causing the intracellular tyrosine residues to be proximate. An arrow points from the second panel to the third panel with text transcribed: Tyrosine residues on the intracellular domain are phosphorylated, triggering a downstream cellular response. The third panel shows the intracellular domains now phosphorylated at several spots represented as green circles with a “P,” indicating activation. A purple arrow (labeled “Cellular response”) points down and away from the third panel. This phosphorylation leads to further downstream signaling cascades inside the cell. [Return to Figure 21.3.2]
Practice Questions
Licenses and Attributions
“Insulin and Glucagon Signaling” by Christelle Sabatier is adapted from “9.1 Signaling Molecules and Cellular Receptors” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e used under CC BY 4.0 and from “Signaling Molecules and Receptors” by OpenStaxCollege for Biology used under CC BY 4.0. “Insulin and Glucagon Signaling” is licensed under CC BY-NC 4.0.
Media Attributions
- 1C_21_GPCR © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- 1C_21_ReceptorTyrosineKinase © OpenStaxCollege is licensed under a CC BY (Attribution) license
