20.3 Propagation of the Signal
Learning Objectives
By the end of this section, you will be able to do the following:
- Explain how the binding of a ligand initiates signal transduction throughout a cell.
- Describe the role of phosphorylation in the transmission of intracellular signals.
- Describe the role of second messengers in signal transmission.
Once a ligand binds to a receptor, the signal is transmitted through the membrane and into the cytoplasm. Continuation of a signal in this manner is called signal transduction. Signal transduction only occurs with cell-surface receptors because internal receptors are able to interact directly with DNA in the nucleus to initiate protein synthesis.
When a ligand binds to its receptor, conformational changes occur that affect the receptor’s intracellular domain. Conformational changes of the extracellular domain upon ligand binding can propagate through the membrane region of the receptor and lead to activation of the intracellular domain or its associated proteins. In some cases, binding of the ligand causes dimerization of the receptor, which means that two receptors bind to each other to form a stable complex called a dimer. A dimer is a chemical compound formed when two molecules (often identical) join together. The binding of the receptors in this manner enables their intracellular domains to come into close contact and activate each other.
Binding Initiates a Signaling Pathway
After the ligand binds to the cell-surface receptor, the activation of the receptor’s intracellular components sets off a chain of events that is called a signaling pathway or a signaling cascade. In a signaling pathway, second messengers, enzymes, and activated proteins interact with specific proteins, which are in turn activated in a chain reaction that eventually leads to a change in the cell’s environment (Figure 20.3.1). The events in the cascade occur in a series, much like a current flows in a river. Interactions that occur before a certain point are defined as upstream events, and events after that point are called downstream events.
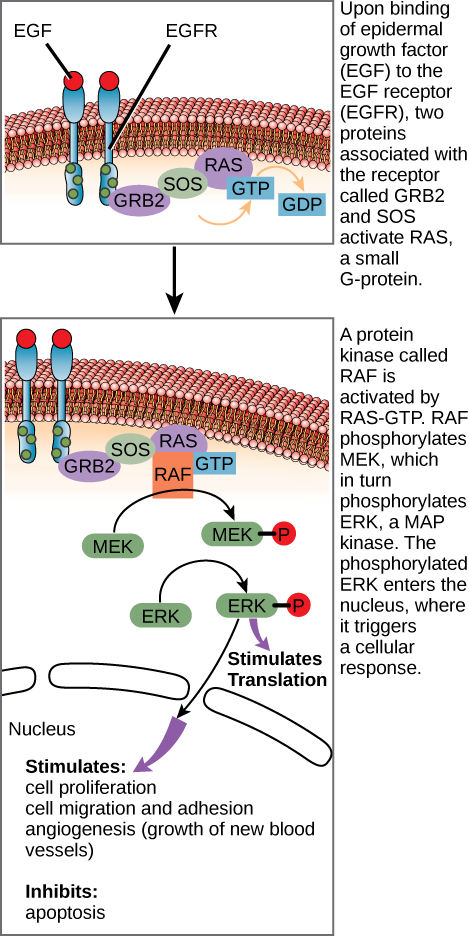
If EGFR is activated at inappropriate times, uncontrolled cell growth (cancer) may occur. In certain cancers, the GTPase activity of the RAS G-protein is inhibited. This means that the RAS protein can no longer hydrolyze GTP into GDP. What effect would this have on downstream cellular events?
Signaling pathways can get very complicated very quickly because most cellular proteins can affect different downstream events, depending on the conditions within the cell. A single pathway can branch off toward different endpoints based on the interplay between two or more signaling pathways, and the same ligands are often used to initiate different signals in different cell types. This variation in response is due to differences in protein expression in different cell types. Another complicating element is signal integration of the pathways, in which signals from two or more different cell-surface receptors merge to activate the same response in the cell. This process can ensure that multiple external requirements are met before a cell commits to a specific response.
The effects of extracellular signals can also be amplified by enzymatic cascades. At the initiation of the signal, a single ligand binds to a single receptor. However, activation of a receptor-linked enzyme can activate many copies of a component of the signaling cascade, which amplifies the signal.
Methods of Intracellular Signaling
The induction of a signaling pathway depends on the modification of a cellular component by an enzyme. There are numerous enzymatic modifications that can occur, and they are recognized in turn by the next component downstream. The following are some of the more common events in intracellular signaling.
Video 20.3.1. Cell Signals (Full length) by DNA Learning Center
Phosphorylation
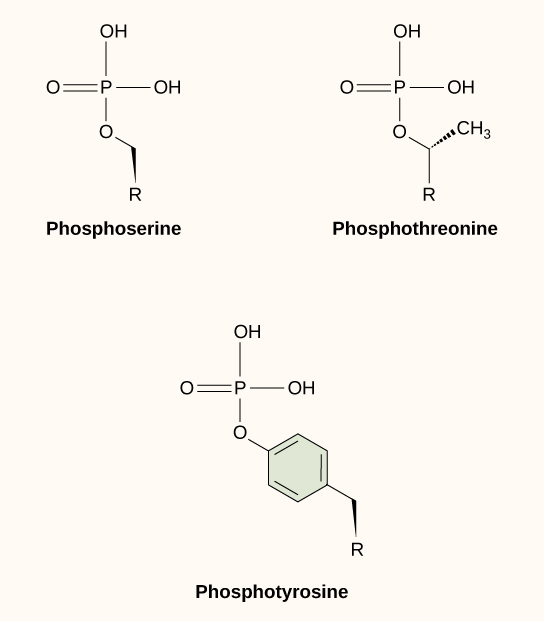
One of the most common chemical modifications that occurs in signaling pathways is the addition of a phosphate group (PO4–3) to a molecule such as a protein in a process called phosphorylation. The phosphate can be added to a nucleotide such as GMP to form GDP or GTP. Phosphates are also often added to serine, threonine, and tyrosine residues of proteins, where they replace the hydroxyl group of the amino acid (Figure 20.3.2). The transfer of the phosphate is catalyzed by an enzyme called a kinase. Various kinases are named for the substrate they phosphorylate. Phosphorylation of serine and threonine residues often activates enzymes. Phosphorylation of tyrosine residues can either affect the activity of an enzyme or create a binding site that interacts with downstream components in the signaling cascade. Phosphorylation may activate or inactivate enzymes, and the reversal of phosphorylation, dephosphorylation by a phosphatase, will reverse the effect.
Second Messengers
Second messengers are small molecules that propagate a signal after it has been initiated by the binding of the signaling molecule to the receptor. These molecules help to spread a signal through the cytoplasm by altering the behavior of certain cellular proteins.
Calcium ion is a widely used second messenger. The free concentration of calcium ions (Ca2+) within a cell is very low because ion pumps in the plasma membrane continuously use adenosine-5′-triphosphate (ATP) to remove it. For signaling purposes, Ca2+ is stored in cytoplasmic vesicles, such as the endoplasmic reticulum, or accessed from outside the cell. When signaling occurs, ligand-gated calcium ion channels allow the higher levels of Ca2+ that are present outside the cell (or in intracellular storage compartments) to flow into the cytoplasm, which raises the concentration of cytoplasmic Ca2+. The response to the increase in Ca2+ varies, depending on the cell type involved. For example, in the β-cells of the pancreas, Ca2+ signaling leads to the release of insulin, and in muscle cells, an increase in Ca2+ leads to muscle contractions.
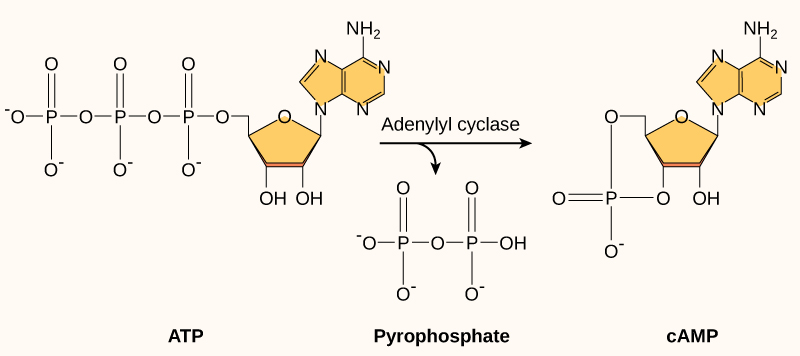
Another second messenger utilized in many different cell types is cyclic AMP (cAMP). Cyclic AMP is synthesized by the enzyme adenylyl cyclase from ATP (Figure 20.3.3). The main role of cAMP in cells is to bind to and activate an enzyme called cAMP-dependent kinase (A-kinase). A-kinase regulates many vital metabolic pathways: It phosphorylates serine and threonine residues of its target proteins, activating them in the process. A-kinase is found in many different types of cells, and the target proteins in each kind of cell are different. Differences give rise to the variation of the responses to cAMP in different cells.
Practice Question
Glossary
cyclic AMP (cAMP)
second messenger that is derived from ATP
cyclic AMP dependent kinase, Protein Kinase A
kinase that is activated by binding to cAMP
diacylglyerol (DAG)
cleavage product of PIP2 that is used for signaling within the plasma membrane
dimer
chemical compound formed when two molecules join together
dimerization of receptor proteins
interaction of two receptor proteins to form a functional complex called a dimer
kinase
enzyme that catalyzes the transfer of a phosphate group from ATP to another molecule
second messenger
small, non-protein molecule that propagates a signal within the cell after activation of a receptor causes its release
signal integration
interaction of signals from two or more different cell-surface receptors that merge to activate the same response in the cell
signal transduction
propagation of the signal through the cytoplasm (and sometimes also the nucleus) of the cell
signaling pathway/cascade
chain of events that occurs in the cytoplasm of the cell to propagate the signal from the plasma membrane to produce a response
Figure Descriptions
Figure 20.3.1 The image consists of a series of diagrams depicting the signaling pathway of the epidermal growth factor receptor (EGFR). It demonstrates the process from receptor activation to cellular response. Top Panel: Shows the EGFR spanning a cellular membrane, with its ligand-binding part outside the cell. EGFR is shown as two structures with red tops, representing receptor dimerization. Below the membrane, GRB2 and SOS proteins are depicted, linking to a RAS protein bound to GDP. An arrow illustrates the exchange of GDP for GTP. Transcribed Text: Upon binding of epidermal growth factor (EGF) to the EGF receptor (EGFR), two proteins associated with the receptor called GRB2 and SOS activate RAS, a small G-protein. Middle Panel: Illustrates the activated RAS-GTP complex signaling to RAF, another protein, leading to subsequent activation of MEK (depicted by an arrow and a phosphorylated symbol). The layers above and below the membrane continue to show GRB2, SOS, and RAS in continuum. Transcribed Text: A protein kinase called RAF is activated by RAS-GTP. RAF phosphorylates MEK, which in turn phosphorylates ERK, a MAP kinase. The phosphorylated ERK enters the nucleus, where it triggers a cellular response. Bottom Panel: Demonstrates activated ERK, indicated by phosphorylation, interacting with proteins for cellular responses such as transcription and apoptosis suppression. An arrow indicates further downstream signaling. Transcribed Text by the arrow: Stimulates: cell proliferation, cell migration and adhesion, angiogenesis (growth of new blood vessels). Inhibits: apoptosis. The diagrams use varied colors like red, green, purple, and orange, with proteins and small molecules labeled. The cellular membrane is depicted in pink and beige. [Return to Figure 20.3.1]
Figure 20.3.2 The image shows the chemical structures of three phosphorylated amino acids: phosphoserine, phosphothreonine, and phosphotyrosine. The structures are arranged in a triangular layout on a light background. Top left is phosphoserine, depicted with a central phosphate group (PO4) bonded to an additional hydroxyl group (OH) and an R group. Top right is phosphothreonine, similar to phosphoserine, but with an extra methyl group (CH3) attached to the carbon chain. Below them is phosphotyrosine, which features a six-carbon benzene ring structure connected to the phosphate group, with an R group extending from it. [Return to Figure 20.3.2]
Figure 20.3.3 The image depicts a biochemical diagram illustrating the formation of cyclic AMP (cAMP) from ATP. On the left, an ATP molecule is shown with three phosphate groups linked in a chain and a ribose sugar attached to an adenine base. The adenine is highlighted in yellow, with the ribose depicted underneath it. An arrow labeled “Adenylyl cyclase” points from the ATP to two products: pyrophosphate and cAMP. Pyrophosphate, in the center, is shown as a pair of phosphate groups linked together, while cAMP on the right retains a single phosphate group that forms a cycle with the ribose sugar and the adenine base. Both adenine bases are colored yellow for emphasis. The diagram is labeled with “ATP” under the ATP structure, “Pyrophosphate” under the central structure, and “cAMP” under the cAMP structure. [Return to Figure 20.3.3]
Licenses and Attributions
“Propagation of the Signal” is adapted from “9.2 Propagation of the Signal” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e used under CC BY 4.0. “Propagation of the Signal” is licensed under CC BY-NC-SA 4.0.
Media Attributions
- 1C_20_EGFsignaling © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- 1C_20_Phosphorylatedaminoacids © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- 1C_20_cAMP © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
