20.2 Signaling in Single-Celled Organisms
Learning Objectives
By the end of this section, you will be able to do the following:
- Differentiate signaling between single-celled and multicellular organisms.
- Describe how single-celled yeasts use cell signaling to communicate with one another.
- Relate the role of quorum sensing to the ability of some bacteria to form biofilms.
Signaling in single-celled organisms primarily focuses on detecting and responding to changes in their immediate environment to survive and reproduce, often relying on relatively simple mechanisms like chemotaxis or quorum sensing. In contrast, multicellular organisms require more complex signaling systems to coordinate activities between diverse cell types, enabling specialized functions and tissue organization. Understanding these differences provides a foundation for exploring how single-celled organisms perceive and react to their surroundings, setting the stage for a deeper look into their unique signaling strategies in this chapter.
Signaling in Bacteria
Signaling in bacteria enables bacteria to monitor extracellular conditions, ensure that there are sufficient amounts of nutrients, and ensure that hazardous situations are avoided. There are circumstances, however, when bacteria communicate with each other.
The first evidence of bacterial communication was observed in a bacterium that has a symbiotic relationship with Hawaiian bobtail squid. When the population density of the bacteria reaches a certain level, specific gene expression is initiated, and the bacteria produce bioluminescent proteins that emit light. Because the number of cells present in the environment (cell density) is the determining factor for signaling, bacterial signaling was named quorum sensing. In politics and business, a quorum is the minimum number of members required to be present to vote on an issue. Dr. Bonnie Bassler, a microbiologist at Princeton University, worked out the mechanism by which quorum sensing works in the bacterium Vibrio fischeri, which have the capacity to produce bioluminescence by expressing the enzyme luciferase. Free-living V. fischeri do not produce luciferase, but V. fischeri living in a symbiotic relationship with the Hawaiian bobtail squid do. The luminescence makes it difficult to see the squid from below because it effectively eliminates its shadow. In return for camouflage, the squid provides food for the bacteria.
Video 20.2.1 How bacteria “talk” – Bonnie Bassler by TED-Ed
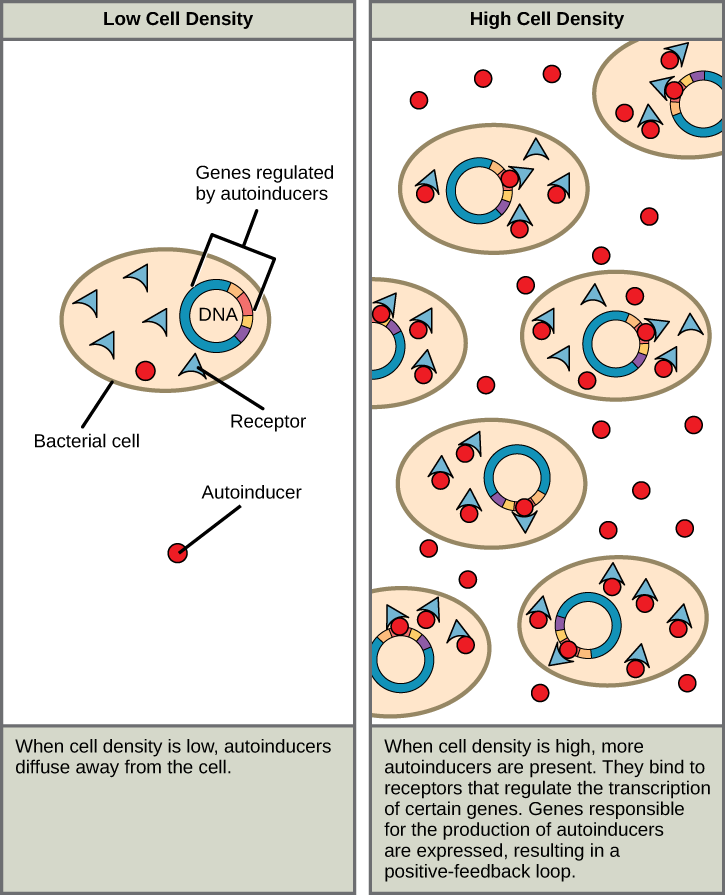
Quorum sensing uses autoinducers as signaling molecules. Autoinducers are signaling molecules secreted by bacteria to communicate with other bacteria of the same kind. The secreted autoinducers can be small, hydrophobic molecules, such as acyl-homoserine lactone (AHL), or larger peptide-based molecules; each type of molecule has a different mode of action. When AHL enters target bacteria, it binds to transcription factors, which then switch gene expression on or off (Figure 20.2.1). The peptide autoinducers stimulate more complicated signaling pathways that include bacterial kinases. The changes in bacteria following exposure to autoinducers can be quite extensive. The pathogenic bacterium Pseudomonas aeruginosa has 616 different genes that respond to autoinducers.
Practice Question
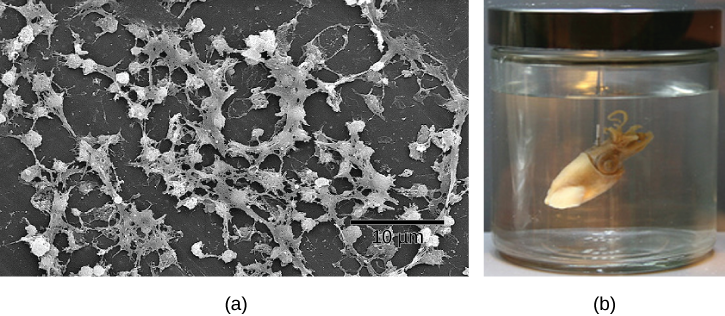
Some species of bacteria that use quorum sensing form biofilms, complex colonies of bacteria (often containing several species) that exchange chemical signals to coordinate the release of toxins that will attack the host. Bacterial biofilms (Figure 20.2.2) can sometimes be found on medical equipment; when biofilms invade implants such as hip or knee replacements or heart pacemakers, they can cause life-threatening infections.
Research on the details of quorum sensing has led to advances in growing bacteria for industrial purposes. Recent discoveries suggest that it may be possible to exploit bacterial signaling pathways to control bacterial growth; this process could replace or supplement antibiotics that are no longer effective in certain situations.
Signaling in Yeast
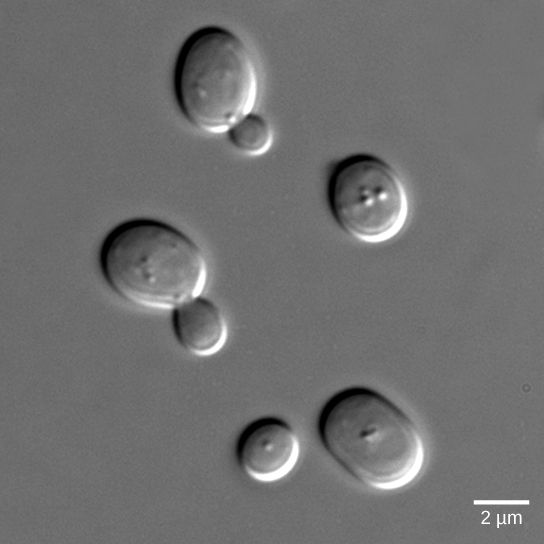
Yeasts are eukaryotes (fungi), and the components and processes found in yeast signals are similar to those of cell-surface receptor signals in multicellular organisms. Budding yeasts (Figure 20.2.3) are able to participate in a process that is similar to sexual reproduction that entails two haploid cells (cells with one-half the normal number of chromosomes) combining to form a diploid cell (a cell with two sets of each chromosome, which is what normal body cells contain). In order to find another haploid yeast cell that is prepared to mate, budding yeasts secrete a signaling molecule called mating factor. When mating factor binds to cell-surface receptors in other yeast cells that are nearby, they stop their normal growth cycles and initiate a cell signaling cascade that includes protein kinases and GTP-binding proteins that are similar to G-proteins (Manning et al, 2002).
Practice Question
Glossary
- autoinducer
- signaling molecule secreted by bacteria to communicate with other bacteria of its kind and others
- mating factor
- signaling molecule secreted by yeast cells to communicate to nearby yeast cells that they are available to mate and communicating their mating orientation
- quorum sensing
- method of cellular communication used by bacteria that informs them of the abundance of similar (or different) bacteria in the environment
Figure Descriptions
Figure 20.2.1. Two-panel diagram. Left panel labeled “Low Cell Density” shows individual bacterial cells with blue triangle-shaped receptors, red circular autoinducer molecules, and DNA strands. Few autoinducers are near cells; they diffuse away. Text reads: “When cell density is low, autoinducers diffuse away from the cell.” Right panel labeled “High Cell Density” shows many bacterial cells close together, surrounded by many autoinducers binding to receptors. Text reads: “When cell density is high, more autoinducers are present. They bind to receptors that regulate the transcription of certain genes. Genes responsible for the production of autoinducers are expressed, resulting in a positive-feedback loop.” [Return to Figure 20.2.1]
Figure 20.2.2. (a) Scanning electron micrograph of Staphylococcus aureus biofilm on a catheter surface, showing dense, irregular clusters of bacterial cells connected by extracellular matrix material. A scale bar reads “10 μm.” (b) Photograph of a Hawaiian bobtail squid suspended in liquid inside a glass jar with a metal lid. [Return to Figure 20.2.2]
Figure 20.2.3. The image displays a microscopic view of several oval-shaped yeast cells. The cells are depicted in gray tones against a slightly darker gray background, showcasing different stages of reproduction. The cells vary in size, with some appearing as single cells while others exhibit budding, indicating cell division. The surface of the cells has a smooth texture, and they appear slightly domed, with subtle highlights indicating the light source. A scale bar is present in the lower right corner, indicating a length of 2 micrometers, providing a size reference for the cells.[Return to Figure 20.2.3]
References
Manning, G., Plowman, G. D., Hunter, T., & Sudarsanam, S. (2002). Evolution of protein kinase signaling from yeast to man. Trends in Biochemical Sciences, 27(10):514–520.
Licenses and Attributions
“Signaling in Single-Celled Organisms” is adapted from “9.4 Signaling in Single-Celled Organisms” by Mary Ann Clark, Matthew Douglas, and Jung Choi for OpenStax Biology 2e used under CC BY 4.0. “Signaling in Single-Celled Organisms” is licensed under CC BY-NC-SA 4.0.
Media Attributions
- 1C_20.2 Quorum sensing © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
- 1C_20.2 Cell-cell communication © a: CDC/Janice Carr; b: Cliff1066/Flickr adapted by OpenStax Biology 2e is licensed under a CC BY-NC (Attribution NonCommercial) license
- 1C_20.1 Budding Yeast © OpenStax Biology 2e is licensed under a CC BY (Attribution) license
