Chapter 14 Acid Base Equilibria
14A (Appendix) Hydrolysis of Salts
Learning Objectives
By the end of this section, you will be able to:
- Describe the acid ionization of hydrated metal ions
Effect of Molecular Structure on Acid-Base Strength
Binary Acids and Bases
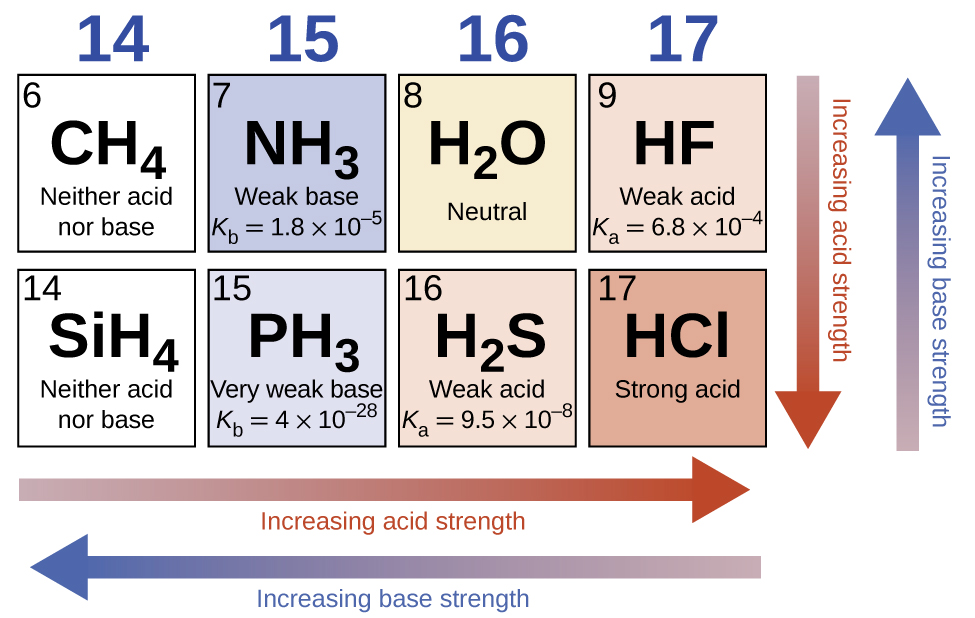
In the absence of any leveling effect, the acid strength of binary compounds of hydrogen with nonmetals (A) increases as the H-A bond strength decreases down a group in the periodic table. For group 17, the order of increasing acidity is HF < HCl < HBr < HI. Likewise, for group 16, the order of increasing acid strength is H2O < H2S < H2Se < H2Te.
Across a row in the periodic table, the acid strength of binary hydrogen compounds increases with increasing electronegativity of the nonmetal atom because the polarity of the H-A bond increases. Thus, the order of increasing acidity (for removal of one proton) across the second row is CH4 < NH3 < H2O < HF; across the third row, it is SiH4 < PH3 < H2S < HCl (see Figure 14.11).
Ternary Acids and Bases
Ternary compounds composed of hydrogen, oxygen, and some third element (“E”) may be structured as depicted in the image below. In these compounds, the central E atom is bonded to one or more O atoms, and at least one of the O atoms is also bonded to an H atom, corresponding to the general molecular formula OmE(OH)n. These compounds may be acidic, basic, or amphoteric depending on the properties of the central E atom. Examples of such compounds include sulfuric acid, O2S(OH)2, sulfurous acid, OS(OH)2, nitric acid, O2NOH, perchloric acid, O3ClOH, aluminum hydroxide, Al(OH)3, calcium hydroxide, Ca(OH)2, and potassium hydroxide, KOH:
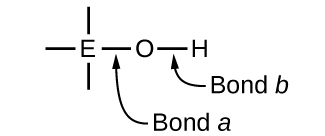
If the central atom, E, has a low electronegativity, its attraction for electrons is low. Little tendency exists for the central atom to form a strong covalent bond with the oxygen atom, and bond a between the element and oxygen is more readily broken than bond b between oxygen and hydrogen. Hence bond a is ionic, hydroxide ions are released to the solution, and the material behaves as a base—this is the case with Ca(OH)2 and KOH. Lower electronegativity is characteristic of the more metallic elements; hence, the metallic elements form ionic hydroxides that are by definition basic compounds.
If, on the other hand, the atom E has a relatively high electronegativity, it strongly attracts the electrons it shares with the oxygen atom, making bond a relatively strongly covalent. The oxygen-hydrogen bond, bond b, is thereby weakened because electrons are displaced toward E. Bond b is polar and readily releases hydrogen ions to the solution, so the material behaves as an acid. High electronegativities are characteristic of the more nonmetallic elements. Thus, nonmetallic elements form covalent compounds containing acidic −OH groups that are called oxyacids.
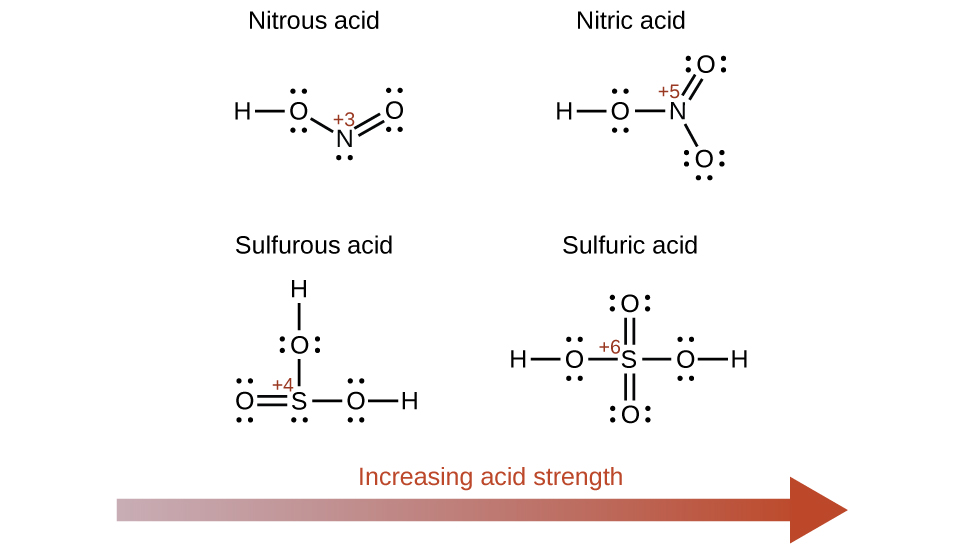
Increasing the oxidation number of the central atom E also increases the acidity of an oxyacid because this increases the attraction of E for the electrons it shares with oxygen and thereby weakens the O-H bond. Sulfuric acid, H2SO4, or O2S(OH)2 (with a sulfur oxidation number of +6), is more acidic than sulfurous acid, H2SO3, or OS(OH)2 (with a sulfur oxidation number of +4). Likewise nitric acid, HNO3, or O2NOH (N oxidation number = +5), is more acidic than nitrous acid, HNO2, or ONOH (N oxidation number = +3). In each of these pairs, the oxidation number of the central atom is larger for the stronger acid (Figure 14.12).
Hydroxy compounds of elements with intermediate electronegativities and relatively high oxidation numbers (for example, elements near the diagonal line separating the metals from the nonmetals in the periodic table) are usually amphoteric. This means that the hydroxy compounds act as acids when they react with strong bases and as bases when they react with strong acids. The amphoterism of aluminum hydroxide, which commonly exists as the hydrate Al(H2O)3(OH)3, is reflected in its solubility in both strong acids and strong bases. In strong bases, the relatively insoluble hydrated aluminum hydroxide, Al(H2O)3(OH)3, is converted into the soluble ion, [Al(H2O)2(OH)4]−, by reaction with hydroxide ion:
In this reaction, a proton is transferred from one of the aluminum-bound H2O molecules to a hydroxide ion in solution. The Al(H2O)3(OH)3 compound thus acts as an acid under these conditions. On the other hand, when dissolved in strong acids, it is converted to the soluble ion [Al(H2O)6]3+ by reaction with hydronium ion:
In this case, protons are transferred from hydronium ions in solution to Al(H2O)3(OH)3, and the compound functions as a base.
The Ionization of Hydrated Metal Ions
Unlike the group 1 and 2 metal ions of the preceding examples (Na+, Ca2+, etc.), some metal ions function as acids in aqueous solutions. These ions are not just loosely solvated by water molecules when dissolved, instead they are covalently bonded to a fixed number of water molecules to yield a complex ion (see chapter on coordination chemistry). As an example, the dissolution of aluminum nitrate in water is typically represented as
However, the aluminum(III) ion actually reacts with six water molecules to form a stable complex ion, and so the more explicit representation of the dissolution process is
As shown in Figure 14.13, the Al(H2O)63+ ions involve bonds between a central Al atom and the O atoms of the six water molecules. Consequently, the bonded water molecules’ O–H bonds are more polar than in nonbonded water molecules, making the bonded molecules more prone to donation of a hydrogen ion:
The conjugate base produced by this process contains five other bonded water molecules capable of acting as acids, and so the sequential or step-wise transfer of protons is possible as depicted in few equations below:
This is an example of a polyprotic acid, the topic of discussion in a later section of this chapter.
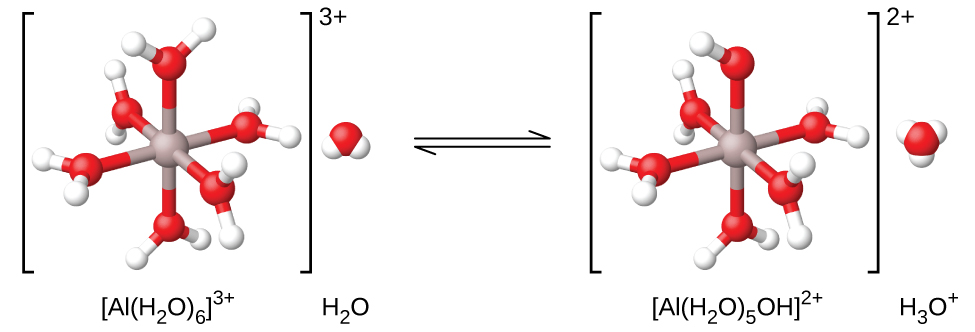
Aside from the alkali metals (group 1) and some alkaline earth metals (group 2), most other metal ions will undergo acid ionization to some extent when dissolved in water. The acid strength of these complex ions typically increases with increasing charge and decreasing size of the metal ions. The first-step acid ionization equations for a few other acidic metal ions are shown below:
Fe(H2O)63+(aq) + H2O(l) ⇌ H3O+(aq) + Fe(H2O)5(OH)2+(aq) pKa = 2.74
Example 14.18 – Hydrolysis of [Al(H2O)6]3+
Calculate the pH of a 0.10-M solution of aluminum chloride, which dissolves completely to give the hydrated aluminum ion [Al(H2O)6]3+ in solution.
Solution
The equation for the reaction and Ka are:
An ICE table with the provided information is
| Al(H2O)63+ | + | H2O | ⇌ | H3O+ | + | Al(H2O)5(OH)2+ | |
| I (M) | 0.10 | — | ~0 | 0 | |||
| C (M) | −x | (−x) | +x | +x | |||
| E (M) | 0.10 − x | — | x | x |
Substituting the expressions for the equilibrium concentrations into the equation for the ionization constant yields:
Assuming x << 0.10 and solving the simplified equation gives:
The ICE table defined x as equal to the hydronium ion concentration, and so the pH is calculated to be
[latex]\text {pH} = - \log {[H_3O^+]} = - \log {(1.2 \times 10^{-3})} = 2.92[/latex]
Check Your Learning
What is [Al(H2O)5(OH)2+] in a 0.15 M solution of Al(NO3)3 that contains enough of the strong acid HNO3 to bring [H3O+] to 0.10 M?
Answer:
2.1 × 10−5 M
Summary
The strengths of the binary acids increase from left to right across a period of the periodic table (CH4 < NH3 < H2O < HF), and they increase down a group (HF < HCl < HBr < HI). The strengths of oxyacids that contain the same central element increase as the oxidation number of the element increases (H2SO3 < H2SO4). The strengths of oxyacids also increase as the electronegativity of the central element increases [H2SeO4 < H2SO4].
Problems
14A.1. Explain why the ionization constant, Ka, for H2SO4 is larger than the ionization constant for H2SO3.
14A.2. Explain why the ionization constant, Ka, for HI is larger than the ionization constant for HF.
14A.3. Predict which acid in each of the following pairs is the stronger and explain your reasoning for each.
(a) H2O or HF
(b) B(OH)3 or Al(OH)3
(c) HSO3− or HSO4−
(d) NH3 or H2S
(e) H2O or H2Te
14A.4. Predict which compound in each of the following pairs of compounds is more acidic and explain your reasoning for each.
(a) HSO4− or HSeO4−
(b) NH3 or H2O
(c) PH3 or HI
(d) NH3 or PH3
(e) H2S or HBr
14A.5. Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
(a) acidity: HCl, HBr, HI
(b) basicity: H2O, OH−, H−, Cl−
(c) basicity: Mg(OH)2, Si(OH)4, ClO3(OH) (Hint: Formula could also be written as HClO4.)
(d) acidity: HF, H2O, NH3, CH4
14A.6. Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
(a) acidity: NaHSO3, NaHSeO3, NaHSO4
(b) basicity: BrO2−, ClO2−, IO2−
(c) acidity: HOCl, HOBr, HOI
(d) acidity: HOCl, HOClO, HOClO2, HOClO3
(e) basicity: NH2−, HS−, HTe−, PH2−
(f) basicity: BrO−, BrO2−, BrO3−, BrO4−
