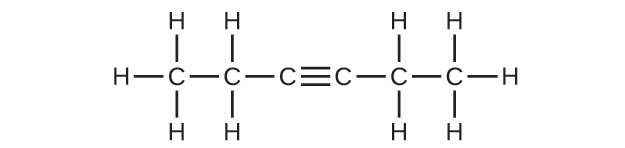Answer Keys to Selected Problems
Chapter 20 Key
20.1. There are several sets of answers; one is:
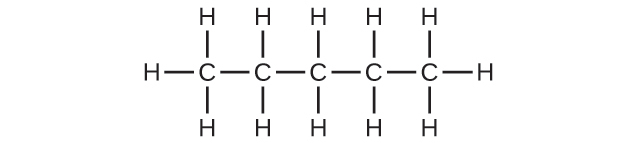
(a) C5H12
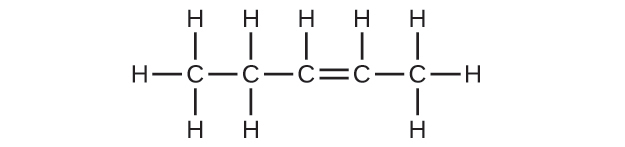
(b) C5H10
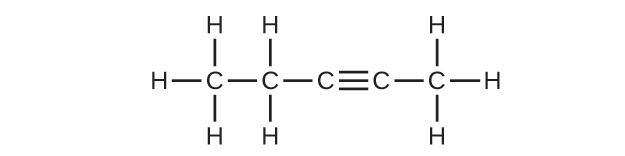
(c) C5H8
20.3. Both reactions result in bromine being incorporated into the structure of the product. The difference is the way in which that incorporation takes place. In the saturated hydrocarbon, an existing C–H bond is broken, and a bond between the C and the Br can then be formed. In the unsaturated hydrocarbon, the only bond broken in the hydrocarbon is the π bond whose electrons can be used to form a bond to one of the bromine atoms in Br2 (the electrons from the Br–Br bond form the other C–Br bond on the other carbon that was part of the π bond in the starting unsaturated hydrocarbon).
20.5. Unbranched alkanes have free rotation about the C–C bonds, yielding all orientations of the substituents about these bonds equivalent, interchangeable by rotation. In the unbranched alkenes, the inability to rotate about the C=C bond results in fixed (unchanging) substituent orientations, thus permitting different isomers. Since these concepts pertain to phenomena at the molecular level, this explanation involves the microscopic domain.
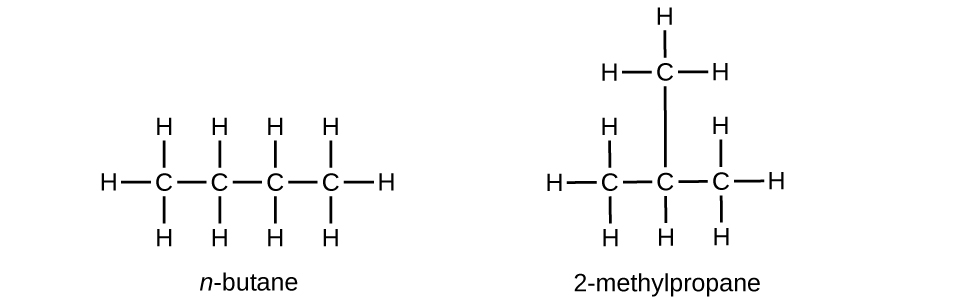
20.7. They are the same compound because each is a saturated hydrocarbon containing an unbranched chain of six carbon atoms.
(a) C6H14
(b) C6H14
(c) C6H12
(d) C6H12
(e) C6H10
(f) C6H10
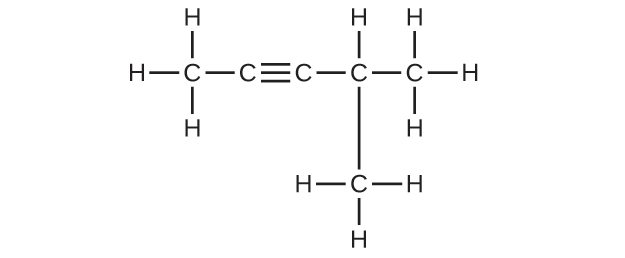
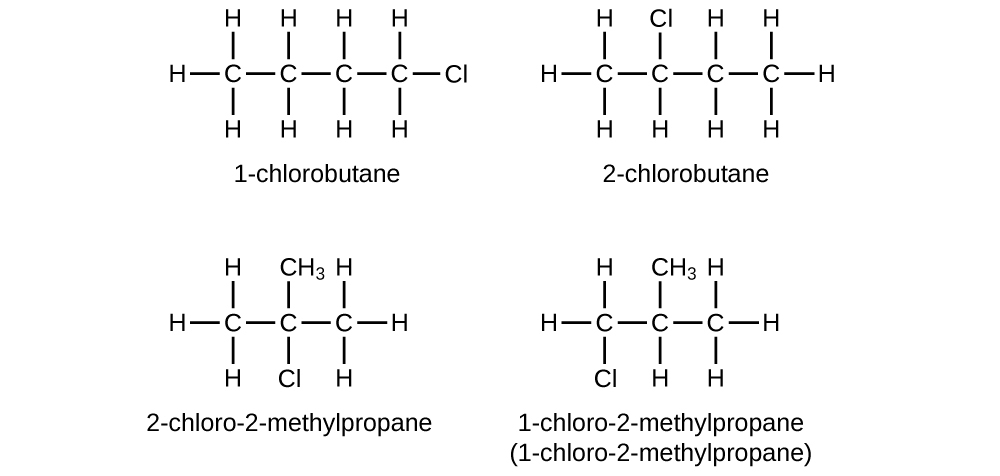
20.11. (a) 2,2-dibromobutane (b) 2-chloro-2-methylpropane (c) 2-methylbutane (d) 1-butyne (e) 4-fluoro-4-methyl-1-octyne (f) trans-1-chloropropene (g) 4-methyl-1-pentene
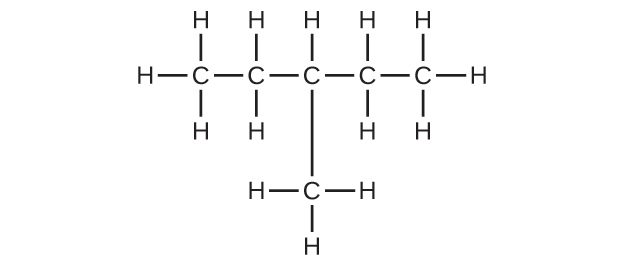
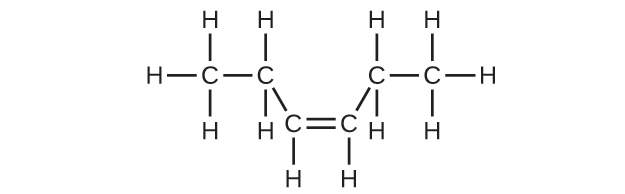
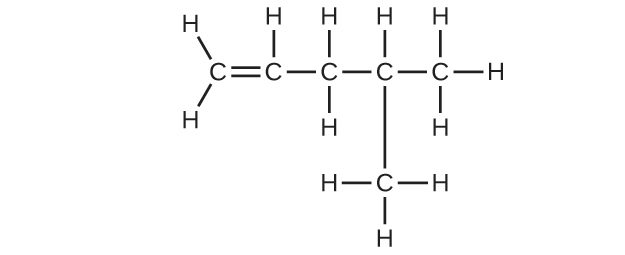
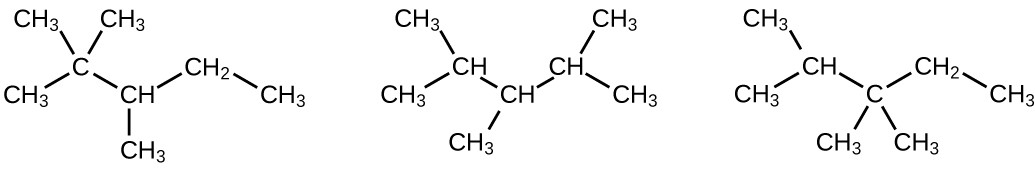
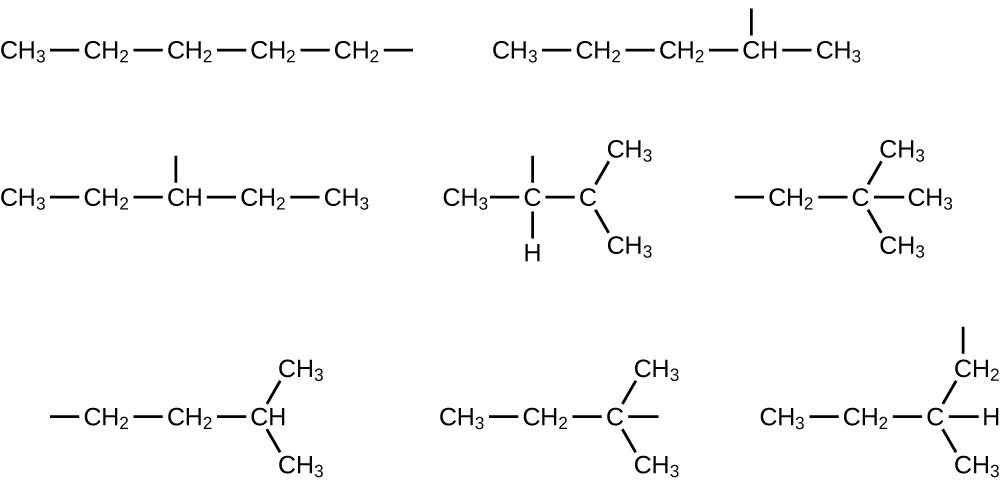
20.17. (a) 2,2,4-trimethylpentane (b) 2,2,3-trimethylpentane, 2,3,4-trimethylpentane, and 2,3,3-trimethylpentane:
20.21. In the following, the carbon backbone and the appropriate number of hydrogen atoms are shown in condensed form:
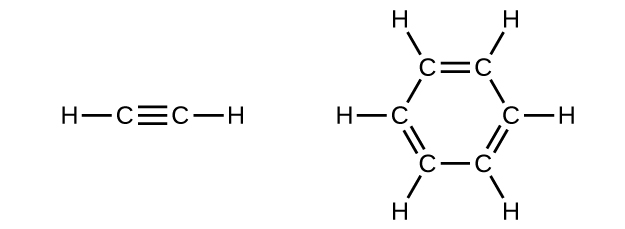
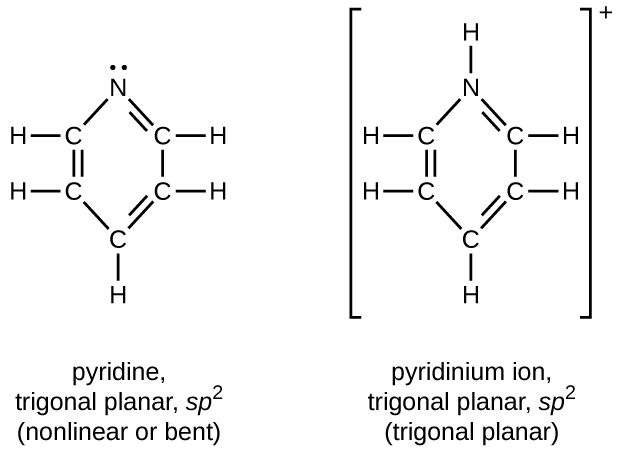
In acetylene, the bonding uses sp hybrids on carbon atoms and s orbitals on hydrogen atoms. In benzene, the carbon atoms are sp2 hybridized.
(a) CH≡CCH2CH3 + 2 I2 → CHI2CI2CH2CH3
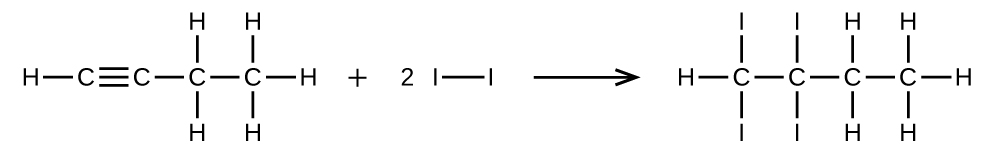
(b) CH3CH2CH2CH2CH3 + 8 O2 → 5 CO2 + 6 H2O
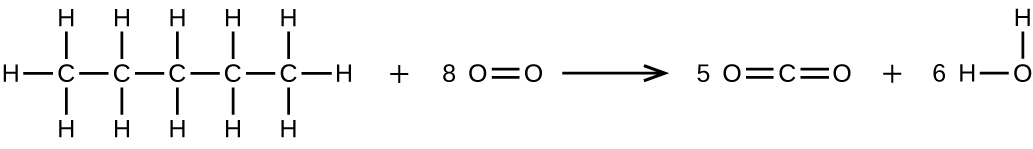
20.31. (a) ethyl alcohol, ethanol: CH3CH2OH (b) methyl alcohol, methanol: CH3OH (c) ethylene glycol, ethanediol: HOCH2CH2OH (d) isopropyl alcohol, 2-propanol: CH3CH(OH)CH3 (e) glycerine, l,2,3-trihydroxypropane: HOCH2CH(OH)CH2OH
20.33. (a) 1-ethoxybutane, butyl ethyl ether (b) 1-ethoxypropane, ethyl propyl ether (c) 1-methoxypropane, methyl propyl ether
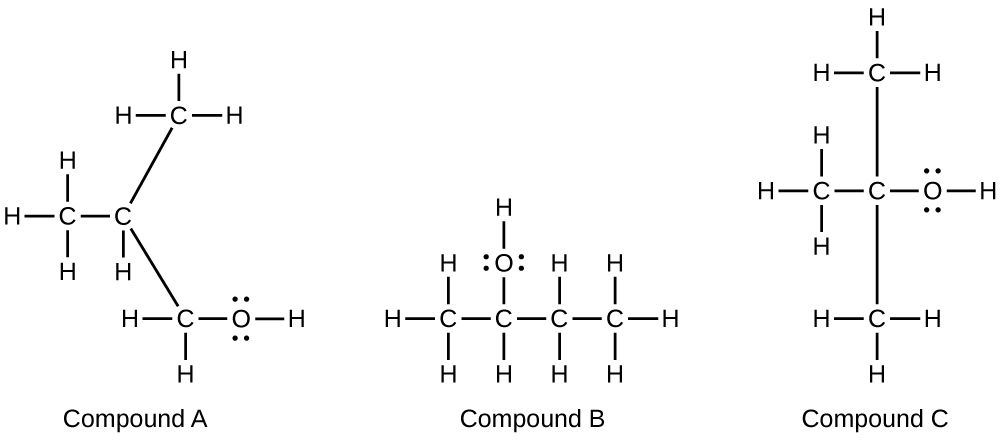
20.35. HOCH2CH2OH, two alcohol groups; CH3OCH2OH, ether and alcohol groups
(a)
(b) 4.593 × 102 L
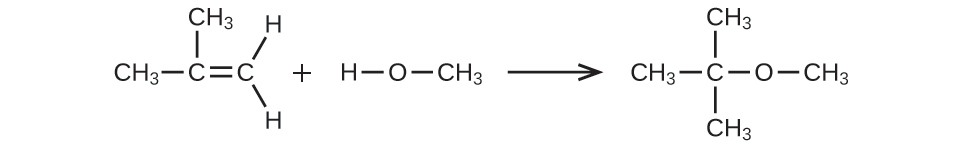
(a) CH3CH=CHCH3 + H2O → CH3CH2CH(OH)CH3
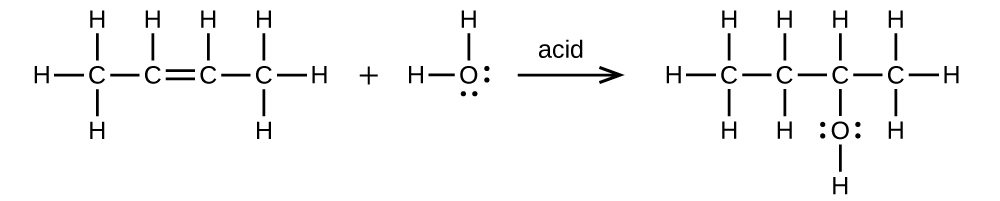
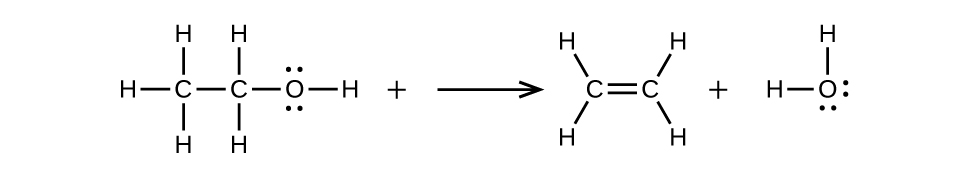
(b) CH3CH2OH → CH2=CH2 + H2O
(a)
(b)
(c)
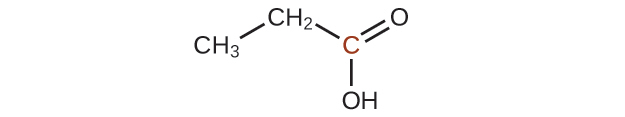
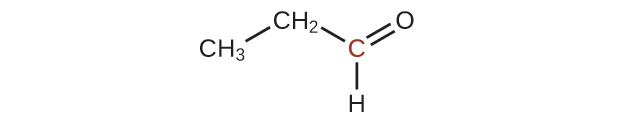
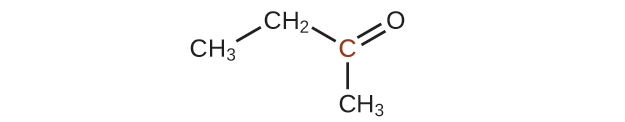
20.43. A ketone contains a group bonded to two additional carbon atoms; thus, a minimum of three carbon atoms are needed.
20.45. Since they are both carboxylic acids, they each contain the –COOH functional group and its characteristics. The difference is the hydrocarbon chain in a saturated fatty acid contains no double or triple bonds, whereas the hydrocarbon chain in an unsaturated fatty acid contains one or more multiple bonds.
(a) CH3CH(OH)CH3: all carbons are tetrahedral
(b) CH3COCH3: the end carbons are tetrahedral and the central carbon is trigonal planar
(c) CH3OCH3: all are tetrahedral
(d) CH3COOH: the methyl carbon is tetrahedral and the acid carbon is trigonal planar
(e) CH3CH2CH2CH(CH3)CHCH2: all are tetrahedral except the right-most two carbons, which are trigonal planar
(a) CH3CH2CH2CH2OH + CH3COOH → CH3COOCH2CH2CH2CH3 + H2O:
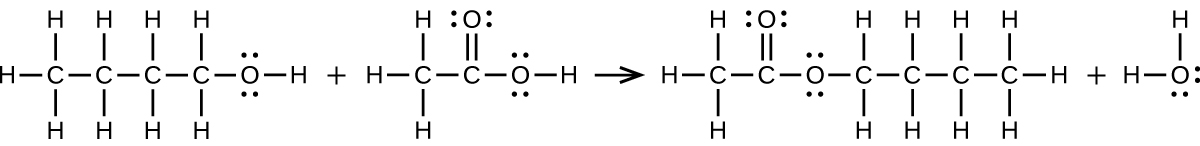
(b) 2 CH3CH2COOH + CaCO3 → CH3CH2COO2Ca + CO2 + H2O:
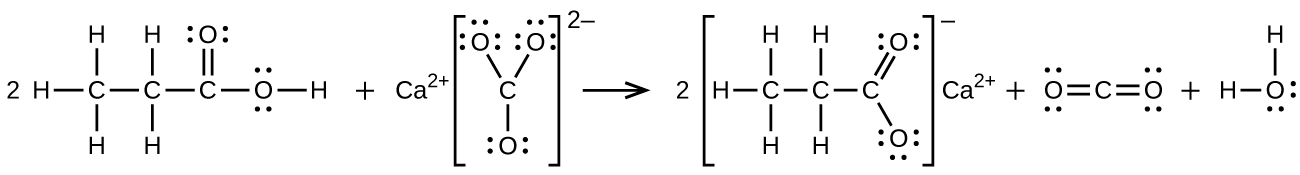
20.55. Trimethyl amine: trigonal pyramidal, sp3; trimethyl ammonium ion: tetrahedral, sp3
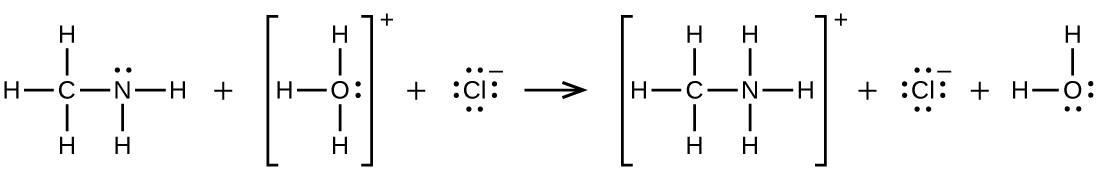
20.59. CH3NH2 + H3O+ → CH3NH3+ + H2O
20.61. CH3CH = CHCH3(sp2) + Cl → CH3CH(Cl)H(Cl)CH3(sp3); 2 C6H6(sp2) + 15 O2 → 12 CO2(sp) + 6 H2O
20.63. The carbon in CO32−, initially at sp2, changes hybridization to sp in CO2.