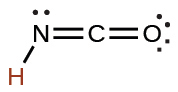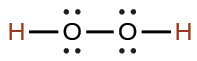Appendix H Ionization Constants of Weak Acids
|
Acid |
Formula |
Ka at 25°C |
Lewis Structure |
|
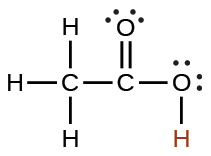
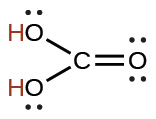
acetic |
CH3CO2H |
1.8 × 10−5 |
|
|
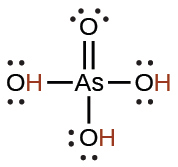
arsenic |
H3AsO4 |
5.5 × 10−3 |
|
|
H2AsO4– |
1.7 × 10−7 |
||
| HAsO42– |
3.0 × 10−12 |
||
|
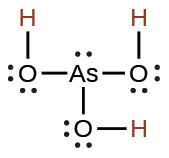
arsenous |
H3AsO3 |
5.1 × 10−10 |
|
|
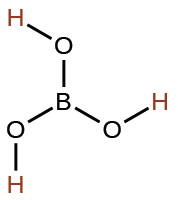
boric |
H3BO3 |
5.4 × 10−10 |
|
|
carbonic |
H2CO3 |
4.3 × 10−7 |
|
| HCO3– |
4.7 × 10−11 |
||
|
cyanic |
HCNO |
2 × 10−4 |
|
|
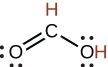
formic |
HCO2H |
1.8 × 10−4 |
|
|
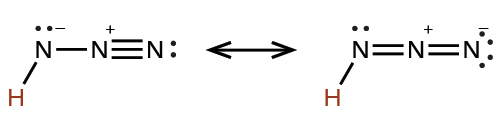
hydrazoic |
HN3 |
2.5 × 10−5 |
|
|
hydrocyanic |
HCN |
4.9 × 10−10 |
|
|
hydrofluoric |
HF |
6.4 × 10−4 |
|
|
hydrogen peroxide |
H2O2 |
2.4 × 10−12 |
|
|
hydrogen selenide |
H2Se |
1.29 × 10−4 |
|
|
HSe– |
1 × 10−12 |
|
|
|
hydrogen sulfate ion |
HSO4– |
1.2 × 10−2 |
|
|
hydrogen sulfide |
H2S |
8.9 × 10−8 |
|
|
HS– |
1.0 × 10−19 |
|
|
|
hydrogen telluride |
H2Te |
2.3 × 10−3 |
|
|
HTe– |
1.6 × 10−11 |
|
|
|
hypobromous |
HBrO |
2.8 × 10−9 |
|
|
hypochlorous |
HClO |
2.9 × 10−8 |
|
|
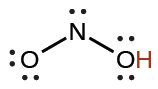
nitrous |
HNO2 |
4.6 × 10−4 |
|
|
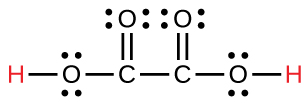
oxalic |
H2C2O4 |
6.0 × 10−2 |
|
|
HC2O4– |
6.1 × 10−5 |
||
|
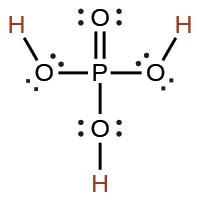
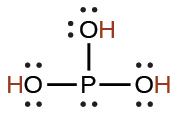
phosphoric |
H3PO4 |
7.5 × 10−3 |
|
|
H2PO4– |
6.2 × 10−8 |
||
|
HPO42– |
4.2 × 10−13 |
||
|
phosphorous |
H3PO3 |
5 × 10−2 |
|
|
H2PO3– |
2.0 × 10−7 |
||
|
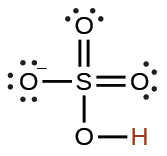
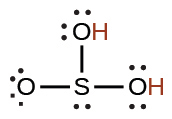
sulfurous |
H2SO3 |
1.6 × 10−2 |
|
| HSO3– |
6.4 × 10−8 |