Chapter 10 Liquids and Solids
Chapter 10 Problems
10.1 Intermolecular Forces
10.1. In terms of their bulk properties, how do liquids and solids differ? How are they similar?
10.2. In terms of the kinetic molecular theory, in what ways are liquids similar to solids? In what ways are liquids different from solids?
10.3. In terms of the kinetic molecular theory, in what ways are liquids similar to gases? In what ways are liquids different from gases?
10.4. Explain why liquids assume the shape of any container into which they are poured, whereas solids are rigid and retain their shape.
10.5. What is the evidence that all neutral atoms and molecules exert attractive forces on each other?
10.6. Open the PhET States of Matter Simulation to answer the following questions:
(a) Select the Solid, Liquid, Gas tab. Explore by selecting different substances, heating and cooling the systems, and changing the state. What similarities do you notice between the four substances for each phase (solid, liquid, gas)? What differences do you notice?
(b) For each substance, select each of the states and record the given temperatures. How do the given temperatures for each state correlate with the strengths of their intermolecular attractions? Explain.
(c) Select the Interaction Potential tab, and use the default neon atoms. Move the Ne atom on the right and observe how the potential energy changes. Select the Total Force button, and move the Ne atom as before. When is the total force on each atom attractive and large enough to matter? Then select the Component Forces button, and move the Ne atom. When do the attractive (van der Waals) and repulsive (electron overlap) forces balance? How does this relate to the potential energy versus the distance between atoms graph? Explain.
10.7. Define the following and give an example of each:
(a) dispersion force
(b) dipole-dipole attraction
(c) hydrogen bond
10.8. The types of intermolecular forces in a substance are identical whether it is a solid, a liquid, or a gas. Why then does a substance change phase from a gas to a liquid or to a solid?
10.9. Why do the boiling points of the noble gases increase in the order He < Ne < Ar < Kr < Xe?
10.10. Neon and HF have approximately the same molecular masses.
(a) Explain why the boiling points of Neon and HF differ.
(b) Compare the change in the boiling points of Ne, Ar, Kr, and Xe with the change of the boiling points of HF, HCl, HBr, and HI, and explain the difference between the changes with increasing atomic or molecular mass.
10.11.
10.12. The molecular mass of butanol, C4H9OH, is 74.14; that of ethylene glycol, CH2(OH)CH2OH, is 62.08, yet their boiling points are 117.2°C and 174°C, respectively. Explain the reason for the difference.
10.13. On the basis of intermolecular attractions, explain the differences in the boiling points of n–butane (−1°C) and chloroethane (12°C), which have similar molar masses.
10.14. On the basis of dipole moments and/or hydrogen bonding, explain in a qualitative way the differences in the boiling points of acetone (56.2°C) and 1-propanol (97.4°C), which have similar molar masses.
10.15. The melting point of H2O(s) is 0°C. Would you expect the melting point of H2S(s) to be −85°C, 0°C, or 185°C? Explain your answer.
10.16. Silane (SiH4), phosphine (PH3), and hydrogen sulfide (H2S) melt at −185°C, −133°C, and −85°C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?
10.17. Explain why a hydrogen bond between two water molecules is weaker than a hydrogen bond between two hydrogen fluoride molecules.
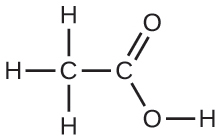
10.18. Under certain conditions, molecules of acetic acid, CH3COOH, form “dimers,” pairs of acetic acid molecules held together by strong intermolecular attractions. Draw a dimer of acetic acid, showing how two CH3COOH molecules are held together, and stating the type of IMF that is responsible.
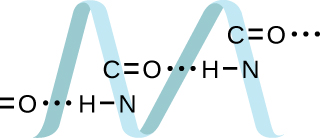
10.19. Proteins are chains of amino acids that can form in a variety of arrangements, one of which is a helix. What kind of IMF is responsible for holding the protein strand in this shape? On the protein image, show the locations of the IMFs that hold the protein together:
10.20. The density of liquid NH3 is 0.64 g/mL; the density of gaseous NH3 at STP is 0.0007 g/mL. Explain the difference between the densities of these two phases.
10.21. Identify the intermolecular forces present in the following solids:
(a) CH3CH2OH
(b) CH3CH2CH3
(c) CH3CH2Cl
10.2 Properties of Liquids
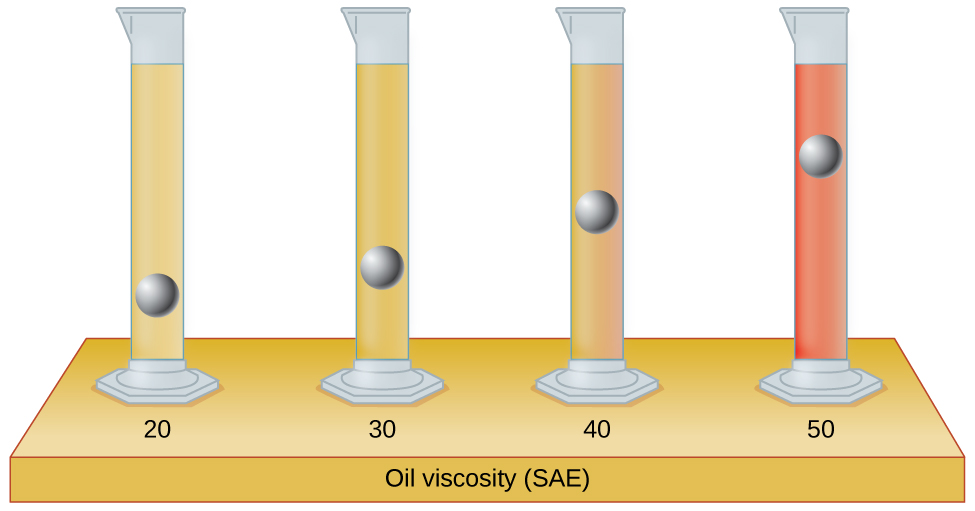
10.22. The test tubes shown here contain equal amounts of the specified motor oils. Identical metal spheres were dropped at the same time into each of the tubes, and a brief moment later, the spheres had fallen to the heights indicated in the illustration. Rank the motor oils in order of increasing viscosity, and explain your reasoning:

10.23. Although steel is denser than water, a steel needle or paper clip placed carefully lengthwise on the surface of still water can be made to float. Explain at a molecular level how this is possible.
10.24. The surface tension and viscosity values for diethyl ether, acetone, ethanol, and ethylene glycol are shown here.
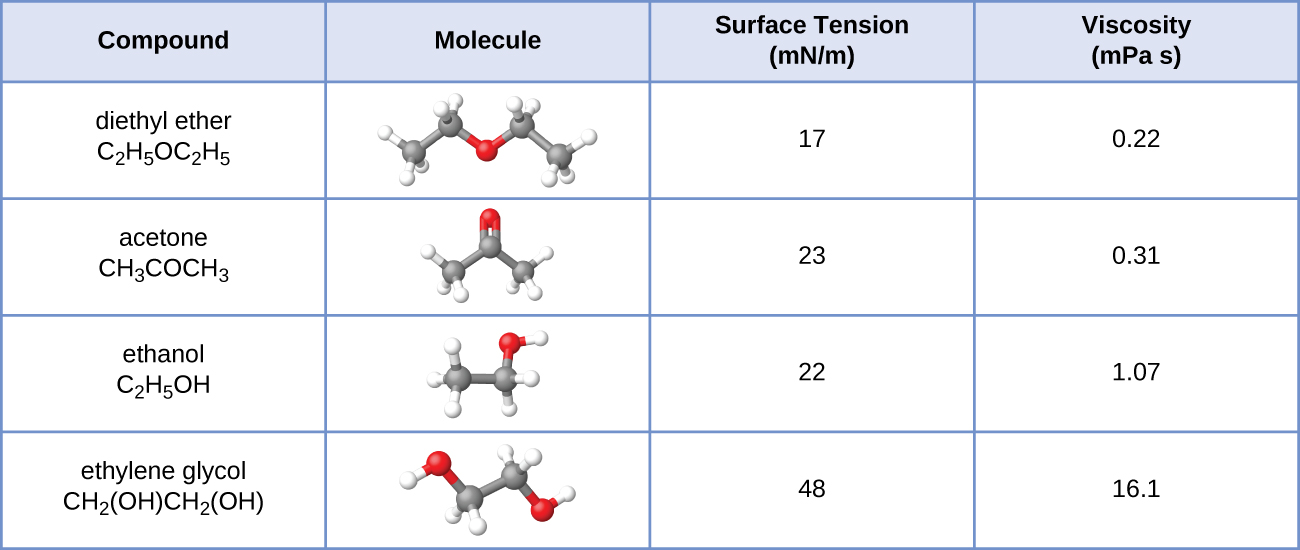
(a) Explain their differences in viscosity in terms of the size and shape of their molecules and their IMFs.
(b) Explain their differences in surface tension in terms of the size and shape of their molecules and their IMFs:
10.25. You may have heard someone use the figure of speech “slower than molasses in winter” to describe a process that occurs slowly. Explain why this is an apt idiom, using concepts of molecular size and shape, molecular interactions, and the effect of changing temperature.
10.26. It is often recommended that you let your car engine run idle to warm up before driving, especially on cold winter days. While the benefit of prolonged idling is dubious, it is certainly true that a warm engine is more fuel efficient than a cold one. Explain the reason for this.
10.27. The surface tension and viscosity of water at several different temperatures are given in this table.
|
Temperature (°C) |
Surface Tension (mN/m) |
Viscosity (mPa s) |
|
0 |
75.6 |
1.79 |
|
20 |
72.8 |
1.00 |
|
60 |
66.2 |
0.47 |
|
100 |
58.9 |
0.28 |
(a) As temperature increases, what happens to the surface tension of water? Explain why this occurs, in terms of molecular interactions and the effect of changing temperature.
(b) As temperature increases, what happens to the viscosity of water? Explain why this occurs, in terms of molecular interactions and the effect of changing temperature.
10.28. At 25°C, how high will water rise in a glass capillary tube with an inner diameter of 0.63 mm? Refer to Example 10.4 for the required information.
10.29. Water rises in a glass capillary tube to a height of 17 cm. What is the diameter of the capillary tube?
10.3 Phase Transitions
10.30. Heat is added to boiling water. Explain why the temperature of the boiling water does not change. What does change?
10.31. Heat is added to ice at 0°C. Explain why the temperature of the ice does not change. What does change?
10.32. What feature characterizes the dynamic equilibrium between a liquid and its vapor in a closed container?
10.33. Identify two common observations indicating some liquids have sufficient vapor pressures to noticeably evaporate?
10.34. Identify two common observations indicating some solids, such as dry ice and mothballs, have vapor pressures sufficient to sublime?
10.35. What is the relationship between the intermolecular forces in a liquid and its vapor pressure?
10.36. What is the relationship between the intermolecular forces in a solid and its melting temperature?
10.37. Why does spilled gasoline evaporate more rapidly on a hot day than on a cold day?
10.38. Carbon tetrachloride, CCl4, was once used as a dry-cleaning solvent, but is no longer used because it is carcinogenic. At 57.8°C, the vapor pressure of CCl4 is 54.0 kPa, and its enthalpy of vaporization is 33.05 kJ/mol. Use this information to estimate the normal boiling point for CCl4.
10.39. When is the boiling point of a liquid equal to its normal boiling point?
10.40. How does the boiling of a liquid differ from its evaporation?
10.41. Use the information in Figure 10.24 to estimate the boiling point of water in Denver when the atmospheric pressure is 83.3 kPa.
10.42. A syringe at a temperature of 20°C is filled with liquid ether in such a way that there is no space for any vapor. If the temperature is kept constant and the plunger is withdrawn to create a volume that can be occupied by vapor, what would be the approximate pressure of the vapor produced?
10.43. Explain the following observations:
(a) It takes longer to cook an egg in Ft. Davis, Texas (altitude, 5000 feet above sea level) than it does in Boston (at sea level).
(b) Perspiring is a mechanism for cooling the body.
10.44. The enthalpy of vaporization of water is larger than its enthalpy of fusion. Explain why.
10.45. Explain why the molar enthalpies of vaporization of the following substances increase in the order CH4 < C2H6 < C3H8, even though the type of IMF (dispersion) is the same.
10.46. Explain why the enthalpies of vaporization of the following substances increase in the order CH4 < NH3 < H2O, even though all three substances have approximately the same molar mass.
10.47. The enthalpy of vaporization of CO2(l) is 9.8 kJ/mol. Would you expect the enthalpy of vaporization of CS2(l) to be 28 kJ/mol, 9.8 kJ/mol, or −8.4 kJ/mol? Discuss the plausibility of each of these answers.
10.48. The hydrogen fluoride molecule, HF, is more polar than a water molecule, H2O (for example, has a greater dipole moment), yet the molar enthalpy of vaporization for liquid hydrogen fluoride is lesser than that for water. Explain.
10.49. Ethyl chloride (boiling point, 13°C) is used as a local anesthetic. When the liquid is sprayed on the skin, it cools the skin enough to freeze and numb it. Explain the cooling effect of liquid ethyl chloride.
10.50. Which contains the compounds listed correctly in order of increasing boiling points?
(a) N2 < CS2 < H2O < KCl
(b) H2O < N2 < CS2 < KCl
(c) N2 < KCl < CS2 < H2O
(d) CS2 < N2 < KCl < H2O
(e) KCl < H2O < CS2 < N2
10.51.
10.52. Evaporation of sweat requires energy and thus takes excess heat away from the body. Some of the water that you drink may eventually be converted into sweat and evaporate. If you drink a 20-ounce bottle of water that has been in the refrigerator at 3.8°C, how much heat is needed to convert all of that water into sweat and then to vapor? (Note: Your body temperature is 36.6°C. For the purpose of solving this problem, assume that the thermal properties of sweat are the same as for water.)
10.53. Titanium tetrachloride, TiCl4, has a melting point of −23.2°C and has a ΔH fusion = 9.37 kJ/mol.
(a) How much energy is required to melt 263.1 g TiCl4?
(b) For TiCl4, which will likely have the larger magnitude: ΔH fusion or ΔH vaporization? Explain your reasoning.
10.4 Phase Diagrams
10.54. From the phase diagram for water (Figure 10.31), determine the state of water at:
(a) 35°C and 85 kPa
(b) −15°C and 40 kPa
(c) −15°C and 0.1 kPa
(d) 75°C and 3 kPa
(e) 40°C and 0.1 kPa
(f) 60°C and 50 kPa
10.55. What phase changes will take place when water is subjected to varying pressure at a constant temperature of 0.005°C? At 40°C? At −40°C?
10.56. Pressure cookers allow food to cook faster because the higher pressure inside the pressure cooker increases the boiling temperature of water. A particular pressure cooker has a safety valve that is set to vent steam if the pressure exceeds 3.4 atm. What is the approximate maximum temperature that can be reached inside this pressure cooker? Explain your reasoning.
10.57. Use the phase diagram for carbon dioxide in Figure 10.34 to answer the following questions:
10.58. Determine the phase changes that carbon dioxide undergoes as pressure is increased at a constant temperature of (a) −50°C and (b) 50°C. If the temperature is held at −40°C? At 20°C? (See the phase diagram in Figure 10.34.)
10.59. Consider a cylinder containing a mixture of liquid carbon dioxide in equilibrium with gaseous carbon dioxide at an initial pressure of 65 atm and a temperature of 20°C. Sketch a plot depicting the change in the cylinder pressure with time as gaseous carbon dioxide is released at constant temperature.
10.60. Dry ice, CO2(s), does not melt at atmospheric pressure. It sublimes at a temperature of −78°C. What is the lowest pressure at which CO2(s) will melt to give CO2(l)? At approximately what temperature will this occur? (See Figure 10.34 for the phase diagram.)
10.61. If a severe storm results in the loss of electricity, it may be necessary to use a clothesline to dry laundry. In many parts of the country in the dead of winter, the clothes will quickly freeze when they are hung on the line. If it does not snow, will they dry anyway? Explain your answer.
10.62. Is it possible to liquefy nitrogen at room temperature (about 25°C)? Is it possible to liquefy sulfur dioxide at room temperature? Explain your answers.
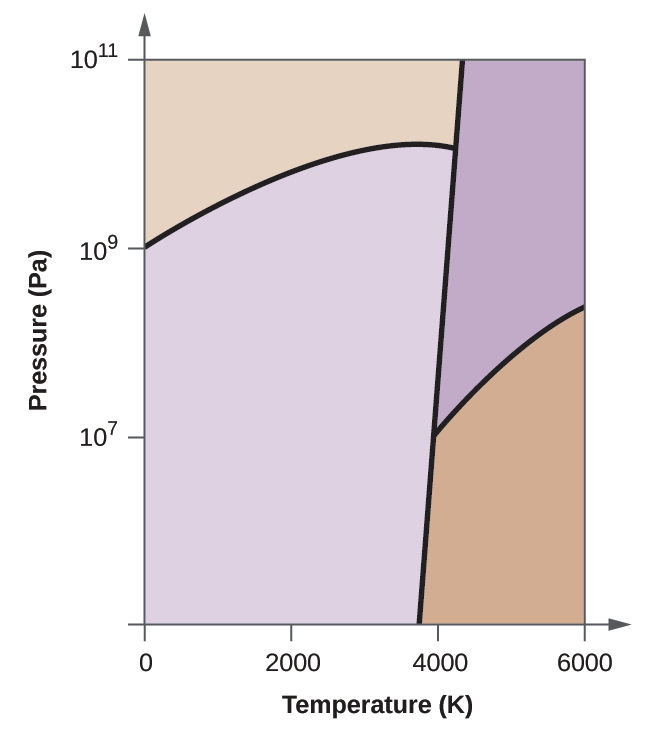
10.63. Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram:
(a) On the phase diagram, label the gas and liquid regions.
(b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
(c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 1010 Pa, it is converted into diamond. Label the diamond phase.
(d) Circle each triple point on the phase diagram.
(e) In what phase does carbon exist at 5000 K and 108 Pa?
(f) If the temperature of a sample of carbon increases from 3000 K to 5000 K at a constant pressure of 106 Pa, which phase transition occurs, if any?
10.5 The Solid State of Matter
10.64. What types of liquids typically form amorphous solids?
10.65. At very low temperatures oxygen, O2, freezes and forms a crystalline solid. Which best describes these crystals?
(a) ionic
(b) covalent network
(c) metallic
(d) amorphous
(e) molecular crystals
10.66. As it cools, olive oil slowly solidifies and forms a solid over a range of temperatures. Which best describes the solid?
(a) ionic
(b) covalent network
(c) metallic
(d) amorphous
(e) molecular crystals
10.67. Explain why ice, which is a crystalline solid, has a melting temperature of 0°C, whereas butter, which is an amorphous solid, softens over a range of temperatures.
10.68. Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by each of the following substances:
(a) SiO2
(b) KCl
(c) Cu
(d) CO2
(e) C (diamond)
(f) BaSO4
(g) NH3
(h) NH4F
(i) C2H5OH
10.69.
10.70. Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid:
|
Substance |
Appearance |
Melting Point |
Electrical Conductivity |
Solubility in Water |
|---|---|---|---|---|
|
X |
lustrous, malleable |
1500°C |
high |
insoluble |
|
Y |
soft, yellow |
113°C |
none |
insoluble |
|
Z |
hard, white |
800°C |
only if melted/dissolved |
soluble |
10.71. Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid:
|
Substance |
Appearance |
Melting Point |
Electrical Conductivity |
Solubility in Water |
|---|---|---|---|---|
|
X |
brittle, white |
800°C |
only if melted/dissolved |
soluble |
|
Y |
shiny, malleable |
1100°C |
high |
insoluble |
|
Z |
hard, colorless |
3550°C |
none |
insoluble |
10.72. Identify the following substances as ionic, metallic, covalent network, or molecular solids:
Substance A is malleable, ductile, conducts electricity well, and has a melting point of 1135°C. Substance B is brittle, does not conduct electricity as a solid but does when molten, and has a melting point of 2072°C. Substance C is very hard, does not conduct electricity, and has a melting point of 3440°C. Substance D is soft, does not conduct electricity, and has a melting point of 185°C.
10.73.
10.74. Substance B is hard, does not conduct electricity, and melts at 1200°C. Substance B is likely a(n):
(a) ionic solid
(b) metallic solid
(c) molecular solid
(d) covalent network solid
Cumulative
10.75. Earth is covered by water; about 70% of Earth’s surface is ocean. In fact, 97% of all water on Earth is salt water.
(a) The average salt concentration of our oceans is 6.0 × 102 mM; what is the concentration in units of g/L? Assume all of the salt is sodium chloride.
(b) Given that water is essential to life and most of the water on Earth has salt in it, drinking water can be made through desalination. One way to desalinate water is to boil off the water vapor (leaving the salt behind) and then condense the vapor into fresh water. How much energy (in kJ) is required to raise the temp of 205 kg of water from 25°C to its boiling point? Use signs to indicate whether heat is absorbed or released. Refer to Appendix E for water properties.
(c) How many kilojoules of energy does it take the change this water (205 kg) to steam? Use signs to indicate whether heat is absorbed or released.
(d) How many kilojoules of energy does it take to change the 205 kg steam back to liquid water so that it can be used for drinking? (Ignore the energy used for electricity—pumping and refrigeration.) Use signs to indicate whether heat is absorbed or released.
