Chapter 11 Solutions and Colloids
11.1 The Dissolution Process
Learning Objectives
By the end of this section, you will be able to:
- Describe the basic properties of solutions and how they form
- Predict whether a given mixture will yield a solution based on molecular properties of its components
- Explain why some solutions either produce or absorb heat when they form
An earlier chapter of this text introduced solutions, defined as homogeneous mixtures of two or more substances. Often, one component of a solution is present at a significantly greater concentration, in which case it is called the solvent. The other components of the solution present in relatively lesser concentrations are called solutes. Sugar is a covalent solid composed of sucrose molecules, C12H22O11. When this compound dissolves in water, its molecules become uniformly distributed among the molecules of water:
The subscript “aq” in the equation signifies that the sucrose molecules are solutes and are therefore individually dispersed throughout the aqueous solution (water is the solvent). Although sucrose molecules are heavier than water molecules, they remain dispersed throughout the solution; gravity does not cause them to “settle out” over time.
Potassium dichromate, K2Cr2O7, is an ionic compound composed of colorless potassium ions, K+, and orange dichromate ions, Cr2O72−. When a small amount of solid potassium dichromate is added to water, the compound dissolves and dissociates to yield potassium ions and dichromate ions uniformly distributed throughout the mixture (Figure 11.2), as indicated in this equation:
As with the mixture of sugar and water, this mixture is also an aqueous solution. Its solutes, potassium and dichromate ions, remain individually dispersed among the solvent (water) molecules.

Link to Learning
Visit this virtual lab to view simulations of the dissolution of common covalent and ionic substances (sugar and salt) in water.
Water is used so often as a solvent that the word solution has come to imply an aqueous solution to many people. However, almost any gas, liquid, or solid can act as a solvent. Many alloys are solid solutions of one metal dissolved in another; for example, US five-cent coins contain nickel dissolved in copper. Air is a gaseous solution, a homogeneous mixture of nitrogen, oxygen, and several other gases. Oxygen (a gas), alcohol (a liquid), and sugar (a solid) all dissolve in water (a liquid) to form liquid solutions. Table 11.1 gives examples of several different solutions and the phases of the solutes and solvents.
|
Solution |
Solute |
Solvent |
|---|---|---|
|
air |
O2(g) |
N2(g) |
|
soft drinks1 |
CO2(g) |
H2O(l) |
|
hydrogen in palladium |
H2(g) |
Pd(s) |
|
rubbing alcohol |
H2O(l) |
C3H8O(l) (2-propanol)
|
|
saltwater |
NaCl(s) |
H2O(l) |
|
brass |
Zn(s) |
Cu(s) |
1 If bubbles of gas are observed within the liquid, the mixture is not homogeneous and thus is not a solution.
Solutions exhibit these defining traits:
- They are homogeneous; after a solution is mixed, it has the same composition at all points throughout (its composition is uniform).
- The physical state of a solution—solid, liquid, or gas—is typically the same as that of the solvent, as demonstrated by the examples in Table 11.1.
- The components of a solution are dispersed on a molecular scale; they consist of a mixture of separated solute particles (molecules, atoms, and/or ions) each closely surrounded by solvent species.
- The dissolved solute in a solution will not settle out or separate from the solvent.
- The composition of a solution, or the concentrations of its components, can be varied continuously (within limits determined by the solubility of the components, discussed in detail later in this chapter).
The Formation of Solutions
The formation of a solution is an example of a spontaneous process, a process that occurs under specified conditions without the requirement of energy from some external source. Sometimes a mixture is stirred to speed up the dissolution process, but this is not necessary; a homogeneous solution will form eventually. The topic of spontaneity is critically important to the study of chemical thermodynamics and is treated more thoroughly in a later chapter of this text. For purposes of this chapter’s discussion, it will suffice to consider two criteria that favor, but do not guarantee, the spontaneous formation of a solution:
- A decrease in the internal energy of the system (an exothermic change, as discussed in the previous chapter on thermochemistry)
- An increased dispersal of matter in the system (which indicates an increase in the entropy of the system, as you will learn about in the later chapter on thermodynamics)
In the process of dissolution, an internal energy change often, but not always, occurs as heat is absorbed or evolved. An increase in matter dispersal always results when a solution forms from the uniform distribution of solute molecules throughout a solvent.
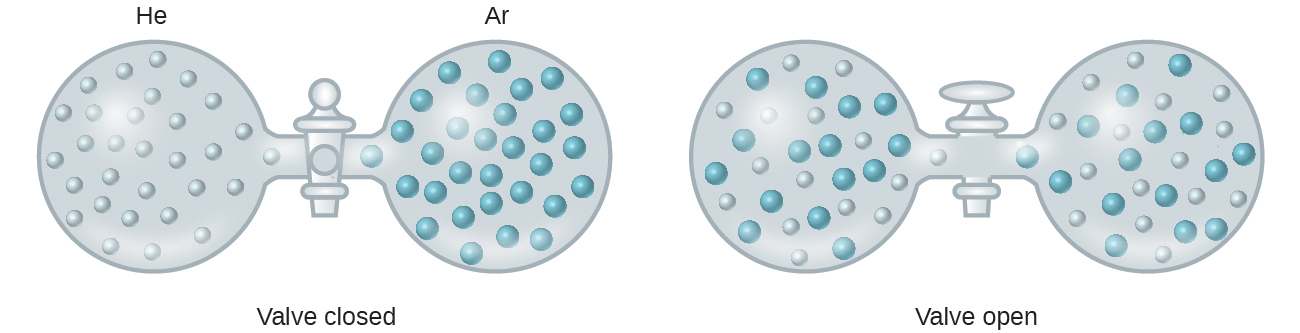
When the strengths of the intermolecular forces of attraction between solute and solvent species in a solution are no different than those present in the separated components, the solution is formed with no accompanying energy change. Such a solution is called an ideal solution. A mixture of ideal gases (or gases such as helium and argon, which closely approach ideal behavior) is an example of an ideal solution, since the entities comprising these gases experience no significant intermolecular attractions.
When containers of helium and argon are connected, the gases spontaneously mix due to diffusion and form a solution (Figure 11.3). The formation of this solution clearly involves an increase in matter dispersal, since the helium and argon atoms occupy a volume twice as large as that which each occupied before mixing.
Ideal solutions may also form when structurally similar liquids are mixed. For example, mixtures of the alcohols methanol (CH3OH) and ethanol (C2H5OH) form ideal solutions, as do mixtures of the hydrocarbons pentane, C5H12, and hexane, C6H14. Placing methanol and ethanol, or pentane and hexane, in the bulbs shown in Figure 11.3 will result in the same diffusion and subsequent mixing of these liquids as is observed for the He and Ar gases (although at a much slower rate), yielding solutions with no significant change in energy. Unlike a mixture of gases, however, the components of these liquid-liquid solutions do, indeed, experience intermolecular attractive forces. But since the molecules of the two substances being mixed are structurally very similar, the intermolecular attractive forces between like and unlike molecules are essentially the same, and the dissolution process, therefore, does not entail any appreciable increase or decrease in energy. These examples illustrate how increased matter dispersal alone can provide the driving force required to cause the spontaneous formation of a solution. In some cases, however, the relative magnitudes of intermolecular forces of attraction between solute and solvent species may prevent dissolution.
Three types of intermolecular attractive forces are relevant to the dissolution process: solute-solute, solvent-solvent, and solute-solvent. As illustrated in Figure 11.4, the formation of a solution may be viewed as a stepwise process in which energy is consumed to overcome solute-solute and solvent-solvent attractions (endothermic processes) and released when solute-solvent attractions are established (an exothermic process referred to as solvation). The relative magnitudes of the energy changes associated with these stepwise processes determine whether the dissolution process overall will release or absorb energy. In some cases, solutions do not form because the energy required to separate solute and solvent species is so much greater than the energy released by solvation.
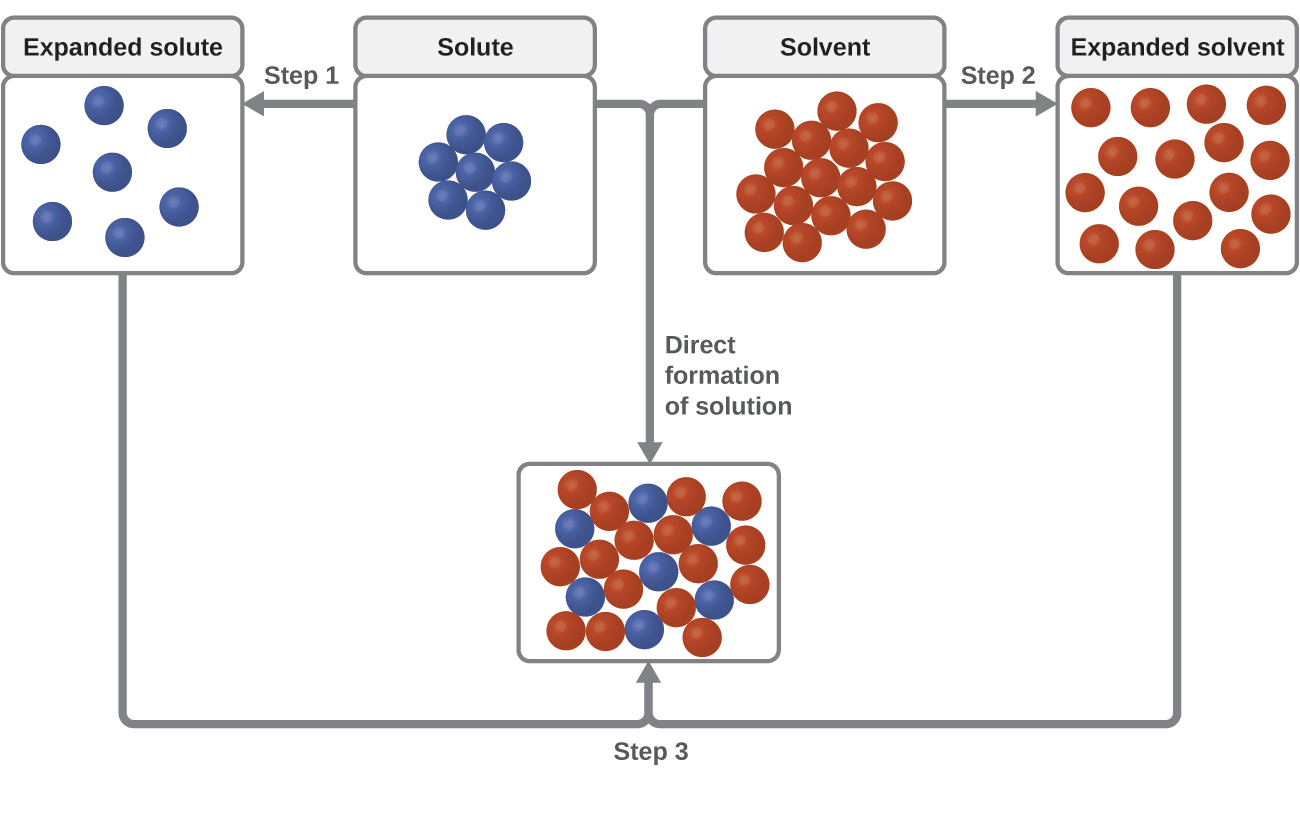
Consider the example of an ionic compound dissolving in water. Formation of the solution requires the electrostatic forces between the cations and anions of the compound (solute–solute) be overcome completely as attractive forces are established between these ions and water molecules (solute–solvent). Hydrogen bonding between a relatively small fraction of the water molecules must also be overcome to accommodate any dissolved solute. If the solute’s electrostatic forces are significantly greater than the solvation forces, the dissolution process is significantly endothermic and the compound may not dissolve to an appreciable extent. Calcium carbonate, the major component of coral reefs, is one example of such an “insoluble” ionic compound (Figure 11.1). On the other hand, if the solvation forces are much stronger than the compound’s electrostatic forces, the dissolution is significantly exothermic and the compound may be highly soluble. A common example of this type of ionic compound is sodium hydroxide, commonly known as lye.
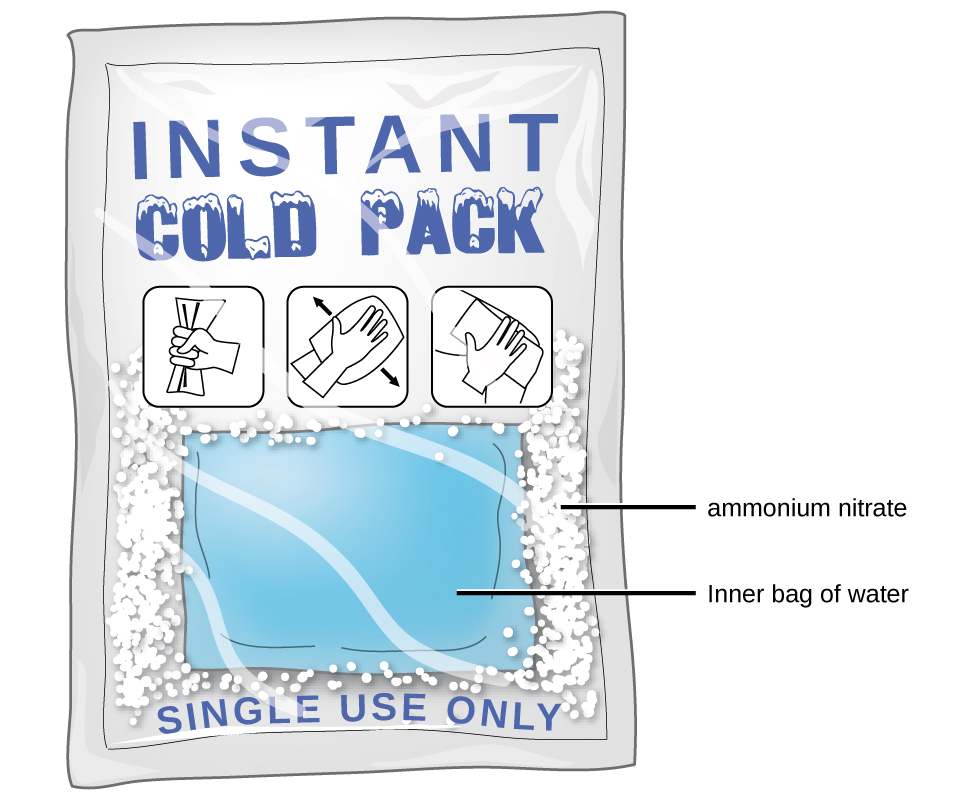
As noted at the beginning of this module, an exothermic dissolution process favors, but is not required for, spontaneous solution formation. While many soluble compounds do, indeed, dissolve with the release of heat, some dissolve endothermically. Ammonium nitrate (NH4NO3) is one such example and is used to make instant cold packs, like the one pictured in Figure 11.5, which are used for treating injuries. A thin-walled plastic bag of water is sealed inside a larger bag with solid NH4NO3. When the smaller bag is broken, a solution of NH4NO3 forms, absorbing heat from the surroundings (the injured area to which the pack is applied) and providing a cold compress that decreases swelling. Endothermic dissolutions such as this one require a greater energy input to separate the solute species than is recovered when the solutes are solvated, but they are spontaneous nonetheless due to the increase in disorder that accompanies formation of the solution.
Link to Learning
Watch this brief video illustrating endothermic and exothermic dissolution processes.
