Chapter 11 Solutions and Colloids
11.3 Solubility
Learning Objectives
By the end of this section, you will be able to:
- Describe the effects of temperature and pressure on solubility
- State Henry’s law and use it in calculations involving the solubility of a gas in a liquid
- Explain the degrees of solubility possible for liquid-liquid solutions
Imagine adding a small amount of sugar to a glass of water, stirring until all the sugar has dissolved, and then adding a bit more. You can repeat this process until the sugar concentration of the solution reaches its natural limit, a limit determined primarily by the relative strengths of the solute-solute, solute-solvent, and solvent-solvent attractive forces discussed in the previous two modules of this chapter. You can be certain that you have reached this limit because, no matter how long you stir the solution, undissolved sugar remains. The concentration of sugar in the solution at this point is known as its solubility.
The solubility of a solute in a particular solvent is the maximum concentration that may be achieved under given conditions when the dissolution process is at equilibrium.
When a solute’s concentration is equal to its solubility, the solution is said to be saturated with that solute. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated. A solution that contains a relatively low concentration of solute is called dilute, and one with a relatively high concentration is called concentrated.
Link to Learning
Use this interactive simulation to prepare various saturated solutions.
Solutions may be prepared in which a solute concentration exceeds its solubility. Such solutions are said to be supersaturated, and they are interesting examples of nonequilibrium states (a detailed treatment of this important concept is provided in later chapters on equilibrium). For example, the carbonated beverage in an open container that has not yet “gone flat” is supersaturated with carbon dioxide gas; given time, the CO2 concentration will decrease until it reaches its saturated (equilibrium) state.
Link to Learning
Watch this impressive video showing the precipitation of sodium acetate from a supersaturated solution.
Solutions of Gases in Liquids
As for any solution, the solubility of a gas in a liquid is affected by the intermolecular attractive forces between solute and solvent species. Unlike solid and liquid solutes, however, there is no solute-solute intermolecular attraction to overcome when a gaseous solute dissolves in a liquid solvent (see Figure 11.4) since the atoms or molecules comprising a gas are far separated and experience negligible interactions. Consequently, solute-solvent interactions are the sole energetic factor affecting solubility.
For example, the water solubility of oxygen is approximately three times greater than that of helium (there are greater dispersion forces between water and the larger oxygen molecules) but 100 times less than the solubility of chloromethane, CHCl3 (polar chloromethane molecules experience dipole–dipole attraction to polar water molecules). Likewise note the solubility of oxygen in hexane, C6H14, is approximately 20 times greater than it is in water because greater dispersion forces exist between oxygen and the larger hexane molecules.
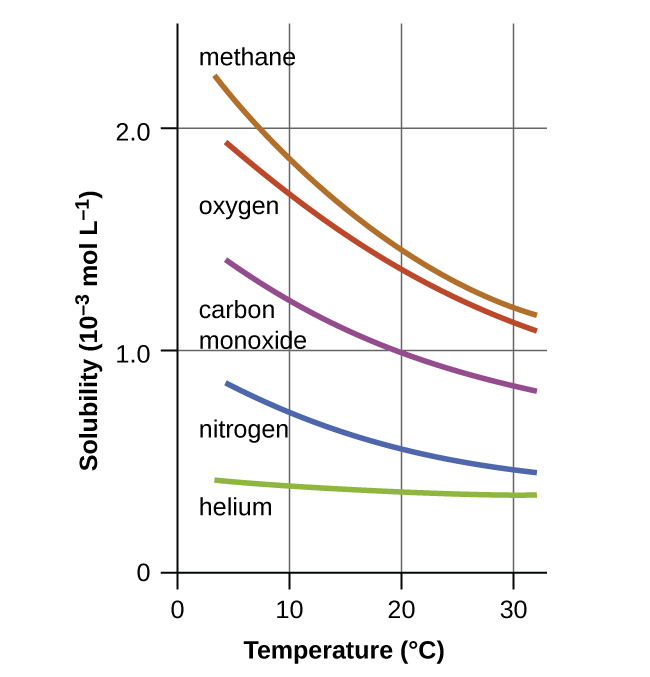
Temperature is another factor affecting solubility, with gas solubility typically decreasing as temperature increases (Figure 11.8). This inverse relation between temperature and dissolved gas concentration is responsible for one of the major impacts of thermal pollution in natural waters. When the temperature of a river, lake, or stream is raised, the solubility of oxygen in the water is decreased. Decreased levels of dissolved oxygen may have serious consequences for the health of the water’s ecosystems and, in severe cases, can result in large-scale fish kills (Figure 11.9).

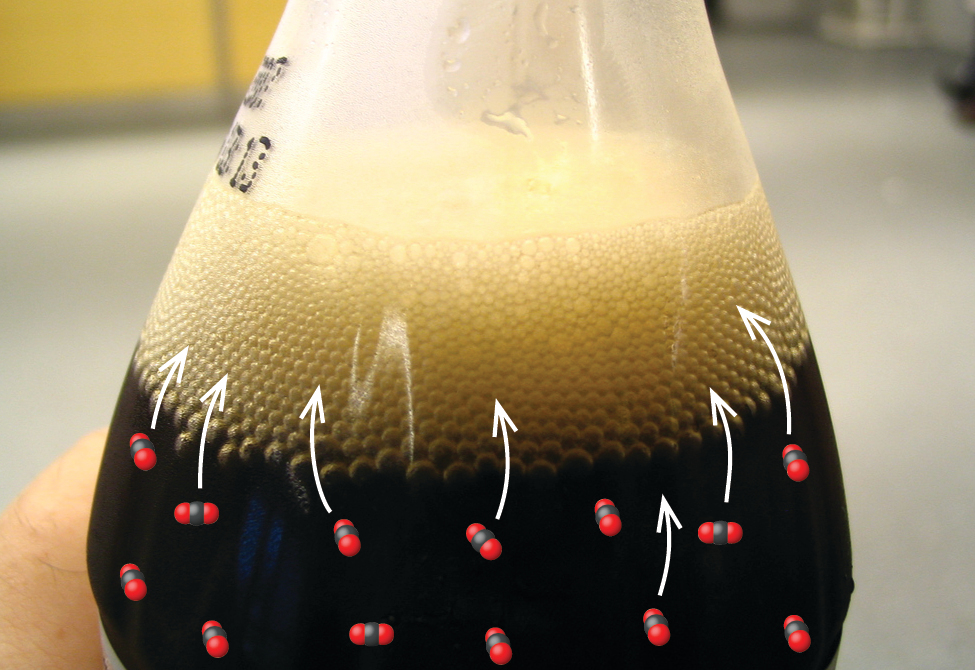
The solubility of a gaseous solute is also affected by the partial pressure of solute in the gas to which the solution is exposed. Gas solubility increases as the pressure of that gas above the solution increases. Carbonated beverages provide a nice illustration of this relationship. The carbonation process involves exposing the beverage to a relatively high pressure of carbon dioxide gas and then sealing the beverage container, thus saturating the beverage with CO2 at this pressure. When the beverage container is opened, a familiar hiss is heard as the carbon dioxide gas pressure is released from the bottle. With the solution now exposed to lower atmospheric CO2 pressures, the beverage is supersaturated with carbon dioxide and, with time, the dissolved carbon dioxide concentration will decrease to its equilibrium value and the beverage will become “flat.” Some of the dissolved carbon dioxide is typically seen leaving solution in the form of small bubbles (Figure 11.10).
For many gaseous solutes, the relation between solubility, Cg, and partial pressure, Pg, is a proportional one:
where k is a proportionality constant that depends on the identity of the gaseous solute, the identity of the solvent, and the solution temperature. This is a mathematical statement of Henry’s law: The quantity of an ideal gas that dissolves in a definite volume of liquid is directly proportional to the pressure of the gas above it.
Example 11.1 – Application of Henry’s Law
At 20°C, the concentration of dissolved oxygen in water exposed to gaseous oxygen at a partial pressure of 101.3 kPa is 1.38 × 10−3 mol L−1. Use Henry’s law to determine the solubility of oxygen when its partial pressure is 20.7 kPa, the approximate pressure of oxygen in earth’s atmosphere.
Solution
According to Henry’s law, for an ideal solution the solubility, Cg, of a gas (1.38 × 10−3 mol L−1, in this case) is directly proportional to the pressure, Pg, of the undissolved gas above the solution (101.3 kPa in this case). Because both Cg and Pg are known, this relation can be rearranged and used to solve for k.
Now, use k to find the solubility at the lower pressure.
Note that various units may be used to express the quantities involved in these sorts of computations. Any combination of units that yield to the constraints of dimensional analysis are acceptable.
Check Your Learning
Click here to see a walkthrough for this problem!
First, we should calculate Cg in units of g/L so we can compare to the answers provided:
Next, we should solve for k in the Henry’s law equation
Finally, we can solve for the Cg under 760 torr of pressure:
Example 11.2 – Thermal Pollution and Oxygen Solubility
A certain species of freshwater trout requires a dissolved oxygen concentration of 7.5 mg/L. Could these fish thrive in a thermally polluted mountain stream (water temperature is 30.0°C, partial pressure of atmospheric oxygen is 0.17 atm)? Use the data in Figure 11.8 to estimate a value for the Henry’s law constant at this temperature.
Solution
First, estimate the Henry’s law constant for oxygen in water at the specified temperature of 30.0°C (Figure 11.8 indicates the solubility at this temperature is approximately ~1.2 mol/L).
Then, use this k value to compute the oxygen solubility at the specified oxygen partial pressure, 0.17 atm.
Finally, convert this dissolved oxygen concentration from mol/L to mg/L.
This concentration is less than the required minimum value of 7.5 mg/L, and so these trout would likely not thrive in the polluted stream.
Check Your Learning
Chemistry in Everyday Life
Decompression Sickness or “The Bends”
Decompression sickness (DCS), or “the bends,” is an effect of the increased pressure of the air inhaled by scuba divers when swimming underwater at considerable depths. In addition to the pressure exerted by the atmosphere, divers are subjected to additional pressure due to the water above them, experiencing an increase of approximately 1 atm for each 10 m of depth. Therefore, the air inhaled by a diver while submerged contains gases at the corresponding higher ambient pressure, and the concentrations of the gases dissolved in the diver’s blood are proportionally higher per Henry’s law.
As the diver ascends to the surface of the water, the ambient pressure decreases and the dissolved gases becomes less soluble. If the ascent is too rapid, the gases escaping from the diver’s blood may form bubbles that can cause a variety of symptoms ranging from rashes and joint pain to paralysis and death. To avoid DCS, divers must ascend from depths at relatively slow speeds (10 or 20 m/min) or otherwise make several decompression stops, pausing for several minutes at given depths during the ascent. When these preventive measures are unsuccessful, divers with DCS are often provided hyperbaric oxygen therapy in pressurized vessels called decompression (or recompression) chambers (Figure 11.11). Researchers are also investigating related body reactions and defenses in order to develop better testing and treatment for decompression sickness. For example, Ingrid Eftedal, a barophysiologist specializing in bodily reactions to diving, has shown that white blood cells undergo chemical and genetic changes as a result of the condition; these can potentially be used to create biomarker tests and other methods to manage decompression sickness.

Deviations from Henry’s law are observed when a chemical reaction takes place between the gaseous solute and the solvent. Thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the law because ammonia, being a base, reacts to some extent with water to form ammonium ions and hydroxide ions.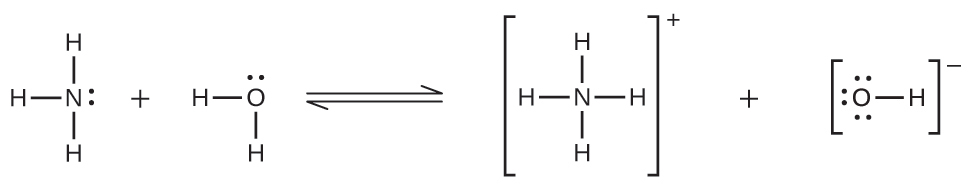
Gases can form supersaturated solutions. If a solution of a gas in a liquid is prepared either at low temperature or under high pressures (or both), then as the solution warms or as the gas pressure is reduced, the solution may become supersaturated. In 1986, more than 1700 people in Cameroon were killed when a cloud of gas, almost certainly carbon dioxide, bubbled from Lake Nyos (Figure 11.12), a deep lake in a volcanic crater. The water at the bottom of Lake Nyos is saturated with carbon dioxide by volcanic activity beneath the lake. It is believed that the lake underwent a turnover due to gradual heating from below the lake, and the warmer, less-dense water saturated with carbon dioxide reached the surface. Consequently, tremendous quantities of dissolved CO2 were released, and the colorless gas, which is denser than air, flowed down the valley below the lake and suffocated humans and animals living in the valley.

Solutions of Liquids in Liquids

Some liquids may be mixed in any proportions to yield solutions; in other words, they have infinite mutual solubility and are said to be miscible. Ethanol, sulfuric acid, and ethylene glycol (popular for use as antifreeze, pictured in Figure 11.13) are examples of liquids that are completely miscible with water. Two-cycle motor oil is miscible with gasoline, mixtures of which are used as lubricating fuels for various types of outdoor power equipment (chainsaws, leaf blowers, and so on).
Miscible liquids are typically those with very similar polarities. Consider, for example, liquids that are polar or capable of hydrogen bonding. For such liquids, the dipole-dipole attractions (or hydrogen bonding) of the solute molecules with the solvent molecules are at least as strong as those between molecules in the pure solute or in the pure solvent. Hence, the two kinds of molecules mix easily. Likewise, nonpolar liquids are miscible with each other because there is no appreciable difference in the strengths of solute-solute, solvent-solvent, and solute-solvent intermolecular attractions. The solubility of polar molecules in polar solvents and of nonpolar molecules in nonpolar solvents is an illustration of the chemical axiom “like dissolves like.”

Two liquids that do not mix to an appreciable extent are called immiscible. Separate layers are formed when immiscible liquids are poured into the same container. Gasoline, oil (Figure 11.14), benzene, carbon tetrachloride, some paints, and many other nonpolar liquids are immiscible with water. Relatively weak attractive forces between the polar water molecules and the nonpolar liquid molecules are not adequate to overcome much stronger hydrogen bonding between water molecules. The distinction between immiscibility and miscibility is really one of extent, so that miscible liquids are of infinite mutual solubility, while liquids said to be immiscible are of very low (though not zero) mutual solubility.
Two liquids, such as bromine and water, that are of moderate mutual solubility are said to be partially miscible. Two partially miscible liquids usually form two layers when mixed. In the case of the bromine and water mixture, the upper layer is water, saturated with bromine, and the lower layer is bromine saturated with water. Since bromine is nonpolar, and, thus, not very soluble in water, the water layer is only slightly discolored by the bright orange bromine dissolved in it. Since the solubility of water in bromine is very low, there is no noticeable effect on the dark color of the bromine layer (Figure 11.15).

Solutions of Solids in Liquids
The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in Figure 11.16. Reviewing these data indicates a general trend of increasing solubility with temperature, although there are exceptions, as illustrated by the ionic compound cerium sulfate.
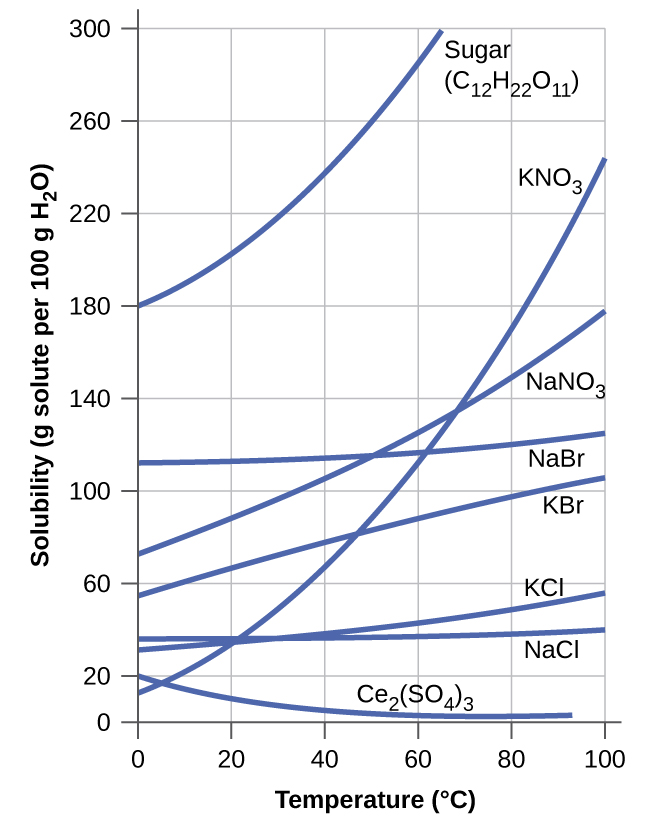
The temperature dependence of solubility can be exploited to prepare supersaturated solutions of certain compounds. A solution may be saturated with the compound at an elevated temperature (where the solute is more soluble) and subsequently cooled to a lower temperature without precipitating the solute. The resultant solution contains solute at a concentration greater than its equilibrium solubility at the lower temperature (i.e., it is supersaturated) and is relatively stable. Precipitation of the excess solute can be initiated by adding a seed crystal (see the video in the Link to Learning earlier in this module) or by mechanically agitating the solution. Some hand warmers, such as the one pictured in Figure 11.17, take advantage of this behavior.

Link to Learning
This video shows the crystallization process occurring in a hand warmer.
