Chapter 11 Solutions and Colloids
11.2 Electrolytes
Learning Objectives
By the end of this section, you will be able to:
- Define and give examples of electrolytes
- Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes
- Relate electrolyte strength to solute-solvent attractive forces
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class of compounds called electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. Solutions can conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Applying a voltage to electrodes immersed in a solution permits assessment of the relative concentration of dissolved ions, either quantitatively, by measuring the electrical current flow, or qualitatively, by observing the brightness of a light bulb included in the circuit (Figure 11.6).
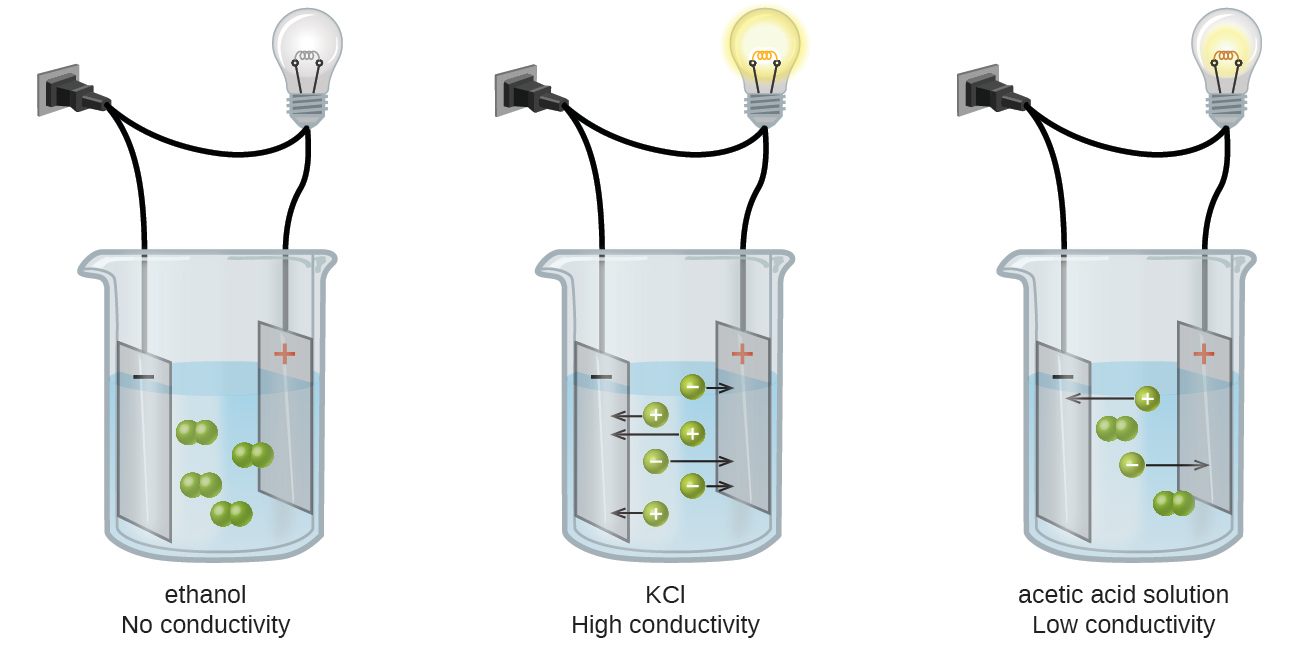
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in Figure 11.7. The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution of ionic compounds in water.
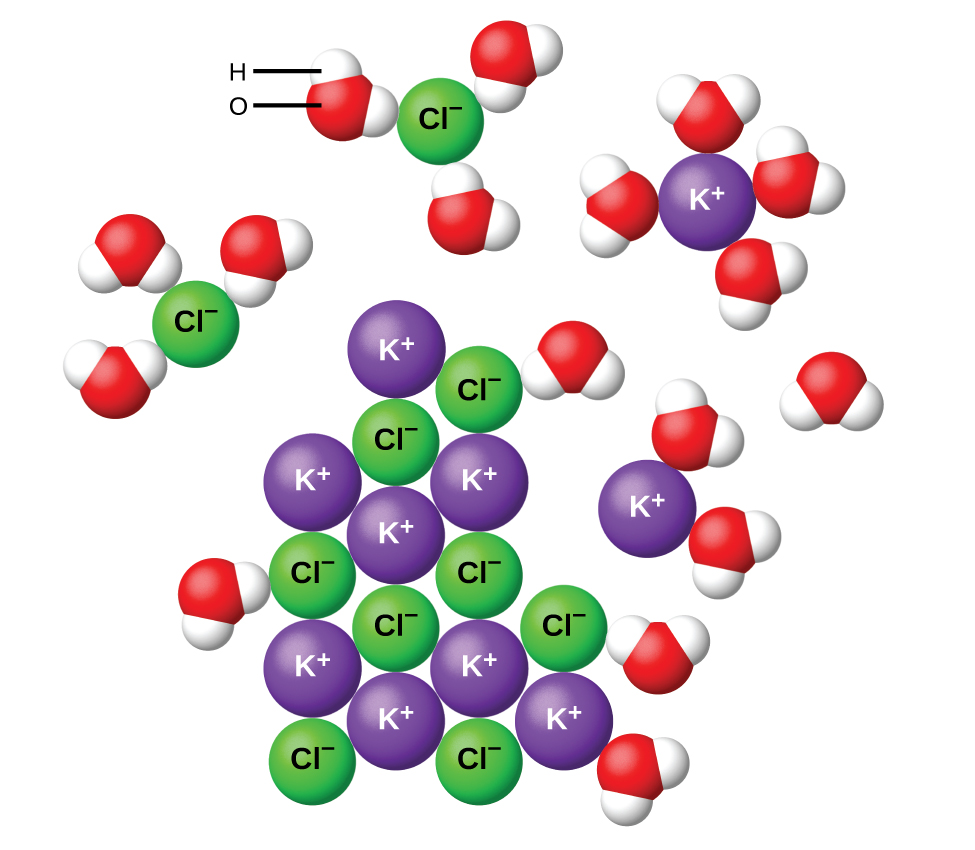
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes. Even ionic compounds that are only slightly soluble are considered strong electrolytes, since the small amount that does dissolve tends to dissociate completely.
Consider what happens at the microscopic level when solid KCl is added to water. Ion-dipole forces attract the positive (hydrogen) end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The water molecules surround individual K+ and Cl− ions, reducing the strong interionic forces that bind the ions together and letting them move off into solution as solvated ions, as Figure 11.7 shows. Overcoming the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution as the ions transition from fixed positions in the undissolved compound to widely dispersed, solvated ions in solution.
One can predict whether an ionic solid will dissolve in water using Tables 11.1A and 11.1B below.
Covalent Electrolytes
Pure water is an extremely poor conductor of electricity because it is only very slightly ionized—only about two out of every 1 billion molecules ionize at 25°C. Water ionizes when one molecule of water gives up a proton (H+ ion) to another molecule of water, yielding hydronium and hydroxide ions, like so:
In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalent HCl molecules. This gas contains no ions. However, an aqueous solution of HCl is a very good conductor, indicating that an appreciable concentration of ions exists within the solution.
Because HCl is an acid, its molecules react with water, transferring H+ ions to form hydronium ions (H3O+) and chloride ions (Cl−):
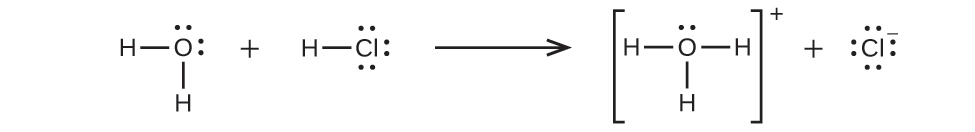
This reaction is essentially 100% complete for HCl (i.e., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of this text on reaction classes and stoichiometry.
Deeper Thinking: Water Filtration Facilities
Water is necessary for life, but it cannot be created in a lab. Have you even stopped to think about how the water you drink, bathe in, and cook with is continuously cycled? How is the water we consume every day produced and safe for consumption? How do water filtration facilities operate?
Chemistry plays a critical role in water treatment. The water cycle is a fundamental tool for evaluating how our environment functions naturally and how humans manipulate this process. Understanding how water treatment works will help you identify relationships and reactions between chemicals. Treatment plants serve as facilities that produce clean drinking water. Water utilities often use a series of water treatment steps that include coagulation, flocculation, sedimentation, filtration, and disinfection. During this process, solution mixing and reaction chemistry are key components. The first step in this process is coagulation, when chemicals such as aluminum sulfate (alum), ferric sulfate, and ferric chloride are added to the unfiltered water. Next, flocculation occurs, which is the gentle mixing of the water so that larger, heavier particles come together. Oftentimes, additional reactive chemicals are added to facilitate this process. The third step is sedimentation, when solids are separated from the water due to floc sedimentation at the bottom of the water. Once the flocs have settled to the bottom of the water, the clear water on top goes through several filters; The filters have different pore (hole) sizes and are made of materials such as sand, gravel, or charcoal. These filters remove germs, including parasites, bacteria, and viruses. They also remove dissolved particles, such as dust and chemicals. Additionally, activated carbon filters remove bad smells. The goal of filtration is for only water, salts, and small charged ions to be present. Disinfection is often the last step. Water treatment plants may add one or more chemical disinfectants to kill any remaining germs. Common types of chemical disinfectants include chlorine, chlorine dioxide, or chloramine. In this step, the pH or acidity of the water is adjusted, and fluorine is added. Adjusting the pH improves taste, reduces corrosion (breakdown) of pipes, and helps chemical disinfectants continue killing germs as the water travels through pipes. Drinking water with an acceptable amount of fluoride keeps teeth strong and reduces cavities. An important note is that water filtration facilities can differ based on the community the water is sourced.
How does water filtration relate to solubility?
- Water filtration’s main purpose is to separate and remove insoluble and soluble impurities. Some examples of soluble compounds are chlorine, dissolved salts, heavy metals, and some organic compounds. Examples of insoluble particles are sand, clay, organic matter, and sediments. In the next section of Chapter 11 and in a later chapter, we will discuss solubility on a deeper level.
Why is this important?
- Overall, this process shows that maintaining clean and unpolluted water sources is critical. It is not an easy step to remove toxic chemicals from water systems; therefore, we need to evaluate how we treat our environment. An input leads to an output, not just in chemical equations, but also in the real world.
