Chapter 11 Solutions and Colloids
11.7 Determining Molar Masses Using Colligative Properties
Learning Objectives
By the end of this section, you will be able to:
- Calculate the molar mass of a compound using experimentally determined colligative properties
Determination of Molar Masses
Osmotic pressure and changes in freezing point, boiling point, and vapor pressure are directly proportional to the number of solute species present in a given amount of solution. Consequently, measuring one of these properties for a solution prepared using a known mass of solute permits determination of the solute’s molar mass.
Example 11.11 - Determination of a Molar Mass from a Freezing Point Depression
A solution of 4.00 g of a nonelectrolyte dissolved in 55.0 g of benzene is found to freeze at 2.32°C. Assuming ideal solution behavior, what is the molar mass of this compound?
Solution
Solve this problem using the following steps. 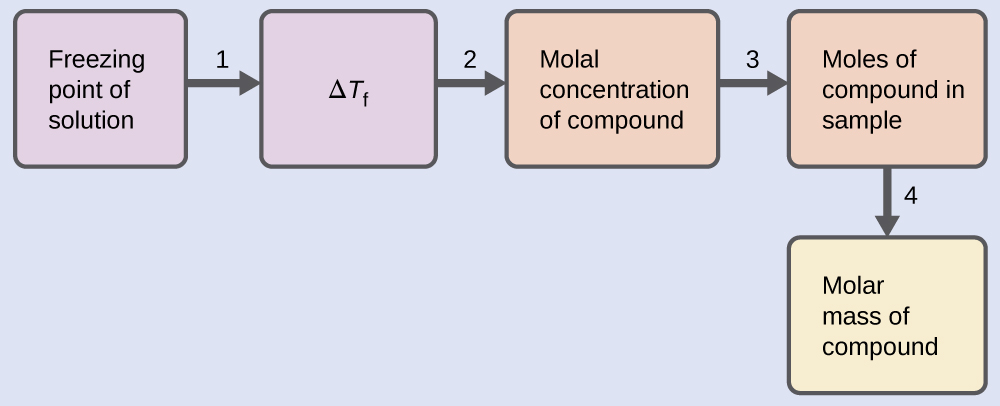
Step 1. Determine the change in freezing point from the observed freezing point and the freezing point of pure benzene (Table 11.2).
Step 2. Determine the molal concentration from Kf, the freezing point depression constant for benzene (Table 11.2), and ΔTf.
Step 3. Determine the number of moles of compound in the solution from the molal concentration and the mass of solvent used to make the solution.
Step 4. Determine the molar mass from the mass of the solute and the number of moles in that mass.
Check Your Learning
Click here for a walkthrough of this problem!
We can find the boiling point (61.26 oC) and boiling point elevation constant (3.63 oC/m) in table 11.2. Then we can calculate the boiling point elevation:
Next, we can solve for the molality of the solution using the boiling point elevation constant:
Next, we use the definition of molality and the mass of the solvent to solve for the mols of solute:
Finally, we can calculate the molar mass using the provided mass of solute:
Example 11.12 - Determination of a Molar Mass from Osmotic Pressure
A 0.500 L sample of an aqueous solution containing 10.0 g of hemoglobin has an osmotic pressure of 5.9 torr at 22°C. Assuming ideal solution behavior, what is the molar mass of hemoglobin?
Solution
Here is one set of steps that can be used to solve the problem: 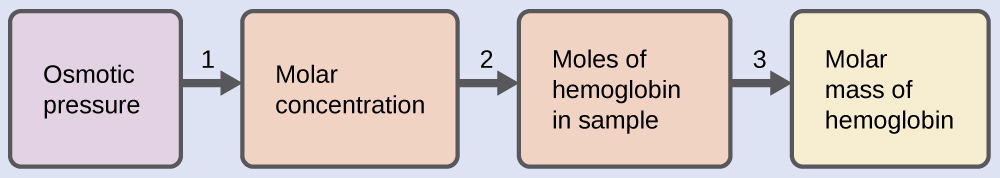
Step 1. Convert the osmotic pressure to atmospheres, then determine the molar concentration from the osmotic pressure.
Step 2. Determine the number of moles of hemoglobin in the solution from the concentration and the volume of the solution.
Step 3. Determine the molar mass from the mass of hemoglobin and the number of moles in that mass.
Check Your Learning
Click here to see a walkthrough for this problem!
First, convert from torr to atm and then calculate the molarity of the solution:
Next, we can use the volume of the solution to calculate the mols of protein from the molarity:
Finally, we can calculate the molar mass of the protein:
