Chapter 14 Acid Base Equilibria
14.4 Structures of Acids and Their Conjugate Bases
Learning Objectives
By the end of this section, you will be able to:
- Identify the most acidic hydrogen from the structure of an acid
- Identify the most basic atom from the structure of a base
- Draw the structure of the conjugate acids and bases
- Predict the products of acid-base reactions
Many compounds, such as acetic acid (CH3COOH), contain many hydrogens, not all of which are acidic. In this section, we will go over how to identify the most acidic hydrogen or most basic atom in a molecule based on its structure. After discussing how to draw the structures of the conjugate acid and base, you will be able to predict the products of acid-base reactions.
Acidic Hydrogens
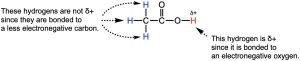
Acidic hydrogens are removed by bases as protons (H+). So the more positively charged the hydrogen is, the more acidic it will be. Hydrogens are typically not fully charged but rather have partial charges, indicated with the symbol δ+. This can occur if hydrogens are bonded to positively charged atoms. This is why ammonium (NH4+) is much more acidic than its neutral analog NH3. Even if the hydrogens are not bonded to positively charged atom, they can still be δ+ if it is participating in polar bonds. For example, in acetic acid, there are hydrogens bonded to a carbon and another bonded an oxygen. Since the C–H bonds are nonpolar while O–H bonds are polar, the hydrogen bonded to the oxygen is more δ+ and thus more acidic (Figure 14.12).
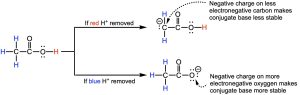
Another way to think about the acidity of compounds is to consider the stability of the conjugate base. The atom that the acidic hydrogen is bonded to becomes negatively charged when the acidic hydrogen is removed. By evaluating how stable this structure is, we can determine how strong of a conjugate base is formed: a stable conjugate base makes it a weak one and a less stable conjugate base a strong one. How does this relate to the acidity? The stronger the acid is, weaker the conjugate base is. Let’s go back to acetic acid. We will remove each possible acidic hydrogen then evaluate the stability of the resulting conjugate base. Let’s first start with one of the hydrogens bonded to the carbon (in blue in Figure 14.13). This results in a conjugate base where the negative charge is on a less electronegative carbon. This negative charge on the carbon results in an unstable conjugate base, i.e. a stronger (conjugate) base. Compare this to removing the hydrogen bonded to the oxygen (in red). This results in a conjugate base where the negative charge is on a much more electronegative oxygen, forming a much more stable conjugate base. The more stable conjugate base means weaker conjugate base, which comes from the stronger acid. So the hydrogen in red, bonded to the more electronegative oxygen, is more acidic.
Example 14.15 – Identifying the Most Acidic Hydrogen in a Compound
Identify the most acidic hydrogen from the following compounds:
(a) 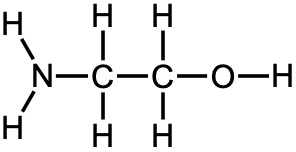
(b) 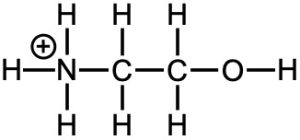
Solution
(a) Since the molecule is neutral, the most acidic hydrogen is the most δ+ one: the one bonded to the most electronegative oxygen.
(b) Since the molecule is positively charged, the hydrogen bonded to the positively charged atom, nitrogen, is most acidic.
Check Your Learning
Identify the most acidic hydrogen from the following pair of compounds:
(a) CH3NH2 and CH3NH3+
(b) H2S and PH3
Solution
(a) The compound that is positively charged, CH3NH3+, is more acidic. Within the compound, the hydrogens bonded to the nitrogen is most acidic, since they are more δ+ than the CH hydrogens.
(b) Since the molecules are neutral, the most acidic hydrogen is the most δ+ one: the one bonded to the more electronegative sulfur. So H2S is more acidic.
Basic Atoms
A base removes an acidic hydrogen from an acid. It does so by forming a bond with its lone pair. So an atom will act as a base, only if it has lone pairs. Since it is grabbing a δ+ hydrogen, the more negatively charged the atom is, the stronger the attraction to the acidic hydrogen. After all, opposites attract! This is why hydroxide (OH−), with its negative charge, is a stronger base than water, where the oxygen is only partially negatively charged (Figure 14.14).

Now let’s compare ammonia and water (Figure 14.15). The nitrogen in the ammonia and the oxygen in water have lone pairs, making these atoms the basic one in each compound. Based on the size of the charge, we might expect water to be the stronger base. However, ammonia is the stronger base. What’s going on? Since neither of the atoms have particularly large charges, we need to consider their electronegativities. Since oxygen is more electronegative, it does not want to share its lone pair with the acidic hydrogen. It would rather keep those lone pair electrons for itself. But, ammonia with the less electronegative nitrogen is more willing to share its lone pair electrons and is the stronger base.
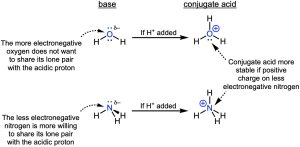
Another way to think about this is to consider the stability of the conjugate acids. Once the oxygen in water and the nitrogen in ammonia are protonated, they will end up with a positive charge (Figure 14.15), and it is better to have a positive charge on a less electronegative atom like nitrogen. In other words, ammonium (NH4+) is more stable or a weaker conjugate acid than hydronium (H3O+), and the weaker the conjugate acid is, the stronger the base it is from.
Example 14.16 – Identifying the Most Basic Atom in a Compound
Identify the most basic atom from the following compounds:
(a) 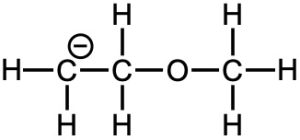
(b) 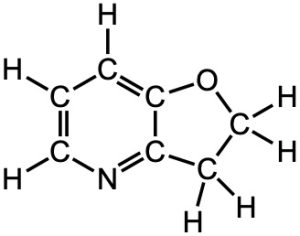
Solution
(a) First, the atom(s) with lone pair(s) should be located, which can accept an acidic hydrogen. In this molecule, negatively charged carbon and the oxygen have lone pairs. In a negative charged compound, the charged atom, carbon in this case, is the most basic atom.
(b) The oxygen and nitrogen have lone pairs. Since neither atoms are charged, the electronegativity should be considered. Since the nitrogen is less electronegative, it is more willing to share its lone pair and is the stronger base.
Check Your Learning
Identify the most basic atom from the following pairs of compounds:
(a) −NH2 and NH3
(b) −OH and −NH2
Solution
(a) In both compounds, the nitrogen has a lone pair and thus will act as the base. Since −NH2 is negatively charged, it is a stronger base than the neutral NH3. So the nitrogen in −NH2 is the most basic atom.
(b) The oxygen in −OH and the nitrogen in −NH2 have lone pairs. Both compounds are negatively charged, so electronegativity should be evaluated next. Because the nitrogen is less electronegative, it is more willing to share its lone pair and is a stronger base.
Predicting the Products of Acid-Base Reactions
As an acid (HA) and base (B) react, the acidic hydrogen is passed from the acid to the base (Figure 14.16). In the acid, the electrons used to retain the acidic hydrogen remains with the acid, now the conjugate base (A−). This decreases the charge of the conjugate base from +1 to 0, 0 to −1, etc. Meanwhile, the base uses a lone pair to bond with the acidic hydrogen to become the conjugate acid (HB+). As it does so, its charge increase from −1 to 0, 0 to +1, etc.

So how can we use this to predict the products of an acid-base reaction? After identifying which compound is the acid and which one the base, the most acidic hydrogen and most basic atom must be identified. Then the acidic hydrogen should be transferred from the acid to the base. We can see this illustrated in Example 14.17.
Example 14.17 – Drawing the Products of an Acid-Base Reaction
Draw the product(s) of the following acid-base reactions.
(a) HNO3 + H2NOH →
(b) CH3CO2H + NH3 →
Solution
Step 1: First step is to identify the acid and the base, and the most acidic hydrogen and the most basic atoms.
(a) HNO3 is a strong acid. Since acids react with bases, H2NOH must be the base in the reaction. Within in HNO3, the only hydrogen present must be the acidic hydrogen. In H2NOH, both the nitrogen and oxygen have lone pairs. However, the less electronegative nitrogen is more willing to share its lone pair. In other words, the nitrogen is the most basic atom.
(b) CH3CO2H has the most polar bond (O−H) of the two compounds. In other words, the OH hydrogen is the most δ+ and is the most acidic. NH3 has the least electronegative atom with a lone pair (N) and will be the base.
Step 2: The acid loses the acidic hydrogen to become the conjugate base, while the base gains the acidic hydrogen to become the conjugate acid.
(a) 
(b) 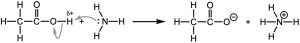
Check Your Learning
Draw the product(s) of the following acid-base reactions.
(a) OH− + H2NNH3+ →
(b) CH3CO2− + HCl →
Solution
(a) H2O + H2NNH2
(b) CH3CO2H + Cl−
Check Your Learning
Which side of the following equilibrium is favored?
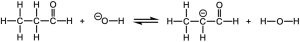
Solution
The side of the equilibrium with the weaker acid and base is favored. The acidities of the acid and the conjugate acid, or the basicities of the base and conjugate base can be evaluated to determine which side has the weaker acid or weaker base. The bases in this case are the negatively charged compounds. Of the two, having the charge on a more electronegative oxygen gives a more stable, weaker base. So the reactant side, which has the weaker base, is favored.
This section introduced you to the basics of identifying the most acidic or basic atom in a molecule. You will see this again next year in Organic Chemistry, where they are an important component of organic reactions!
Media Attributions
- F14.11
- F14.12
- Ex14.15a
- Ex14.15b
- F14.13
- F14.14
- Ex14.16a
- Ex14.16b
- F14.15
- Ex14.17a
- Ex14.17b
- Ex14.17c
