Chapter 15 Equilibria of Other Reaction Classes
15.1 Solubility Product
Learning Objectives
By the end of this section, you will be able to:
- Write chemical equations and equilibrium expressions representing solubility equilibria
- Calculate the solubility of compounds using the solubility product
Solubility equilibria are established when the dissolution and precipitation of a solute species occur at equal rates. These equilibria underlie many natural and technological processes, ranging from tooth decay to water purification. An understanding of the factors affecting compound solubility is, therefore, essential to the effective management of these processes. This section applies previously introduced equilibrium concepts and tools to systems involving dissolution and precipitation.
The Solubility Product
Section 11.3 discussed that the solubility of a substance can vary from essentially zero (insoluble or sparingly soluble) to infinity (miscible). A solute with finite solubility can yield a saturated solution when it is added to a solvent in an amount exceeding its solubility, resulting in a heterogeneous mixture of the saturated solution and the excess, undissolved solute. For example, a saturated solution of silver chloride is one in which the equilibrium shown below has been established.
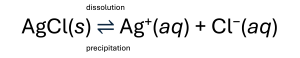
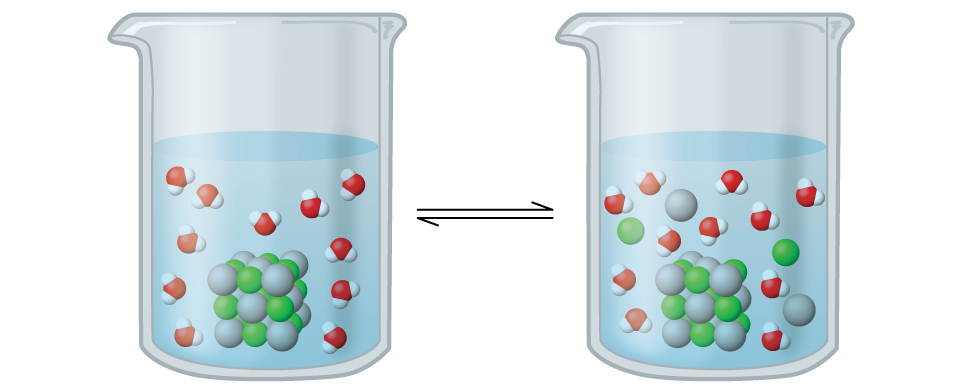
In this solution, an excess of solid AgCl dissolves and dissociates to produce aqueous Ag+ and Cl– ions at the same rate that these aqueous ions combine and precipitate to form solid AgCl (Figure 15.2). Because silver chloride is a sparingly soluble salt, the equilibrium concentration of its dissolved ions in the solution is relatively low.
The equilibrium constant for solubility equilibria such as this one is called the solubility product constant, Ksp, in this case:
AgCl(s) ⇌ Ag+(aq) + Cl−(aq)
Recall that only gases and solutes are represented in equilibrium constant expressions, so the Ksp does not include a term for the undissolved AgCl. A listing of solubility product constants for several sparingly soluble compounds is provided in Appendix J.
Example 15.1 – Writing Equations and Solubility Products
Write the dissolution equation and the solubility product expression for each of the following slightly soluble ionic compounds:
(a) AgI, silver iodide, a solid with antiseptic properties
(b) CaCO3, calcium carbonate, the active ingredient in many over-the-counter chewable antacids
(c) Mg(OH)2, magnesium hydroxide, the active ingredient in Milk of Magnesia
(d) Mg(NH4)PO4, magnesium ammonium phosphate, an essentially insoluble substance used in tests for magnesium
(e) Ca5(PO4)3OH, the mineral apatite, a source of phosphate for fertilizers
Solution
(a) AgI(s) ⇌ Ag+(aq) + I−(aq)
(b) CaCO3(s) ⇌ Ca2+(aq) + CO32−(aq)
(c) Mg(OH)2(s) ⇌ Mg2+(aq) + 2 OH−(aq)
(d) Mg(NH4)PO4(s) ⇌ Mg2+(aq) + NH4+(aq) + PO43−(aq)
(e) Ca5(PO4)3OH(s) ⇌ 5 Ca2+(aq) + 3 PO43−(aq) + OH−(aq)
Check Your Learning
Click here to see the dissolution equations and solubility products for these compounds.
(1) BaSO4(s) ⇌ Ba2+(aq) + SO42−(aq)
(2) Ag2SO4(s) ⇌ 2 Ag+(aq) + SO42−(aq)
(3) Al(OH)3(s) ⇌ Al3+(aq) + 3 OH−(aq)
(4) Pb(OH)Cl(s) ⇌ Pb2+(aq) + OH−(aq) + Cl−(aq)
Ksp and Solubility
The Ksp of a slightly soluble ionic compound may be simply related to its measured solubility provided the dissolution process involves only dissociation and solvation, for example:
For cases such as these, one may derive Ksp values from provided solubilities, or vice-versa. Calculations of this sort are most conveniently performed using a compound’s molar solubility, measured as moles of dissolved solute per liter, in other words in molarity, of saturated solution.
Example 15.2 – Calculation of Ksp from Equilibrium Concentrations
Fluorite, CaF2, is a slightly soluble solid that dissolves according to the equation:
The concentration of Ca2+ in a saturated solution of CaF2 is 2.15 × 10–4 M. What is the solubility product of fluorite?
Solution
According to the stoichiometry of the dissolution equation, the fluoride ion molarity of a CaF2 solution is equal to twice its calcium ion molarity:
Substituting the ion concentrations into the Ksp expression gives:
Check Your Learning
Click here for the solution!
According to the stoichiometry of the dissolution equation:
Substituting the ion concentrations into the Ksp expression gives:
Example 15.3 – Determination of Molar Solubility from Ksp
The Ksp of copper(I) bromide, CuBr, is 6.3 × 10–9. Calculate the molar solubility of copper bromide.
Solution
The dissolution equation and solubility product expression are:
Following the ICE approach to this calculation yields the table:
| CuBr(s) | ⇌ | Cu+(aq) | + | Br−(aq) | |
| I (M) | — | 0 | 0 | ||
| C (M) | (−x) | +x | +x | ||
| E (M) | — | x | x |
Substituting the equilibrium concentration terms into the solubility product expression and solving for x yields:
Since the dissolution stoichiometry shows one mole of copper(I) ion and one mole of bromide ion are produced for each moles of CuBr dissolved, the molar solubility of CuBr is 7.9 × 10–5 M.
Check Your Learning
Click here for a walkthrough!
The dissolution equation and solubility product expression are:
Following the ICE approach to this calculation yields the table:
| AgI(s) | ⇌ | Ag+ (aq) | + | I– (aq) | |
| I (M) | — | 0 | 0 | ||
| C (M) | (−x) | +x | +x | ||
| E (M) | — | x | x |
Substituting the equilibrium concentration terms into the solubility product expression and solving for x yields:
Since the dissolution stoichiometry shows one mole of silver ion and one mole of iodide ion are produced for each mole of AgI dissolved, the molar solubility of AgI is 1.2 × 10–8M.
Example 15.4 – Determination of Molar Solubility from Ksp
The Ksp of calcium hydroxide, Ca(OH)2, is 1.3 × 10–6. Calculate the molar solubility of calcium hydroxide.
Solution
The dissolution equation and solubility product expression are:
The ICE table for this system is:
| Ca(OH)2(s) | ⇌ | Cu2+(aq) | + | 2 OH−(aq) | |
| I (M) | — | 0 | 0 | ||
| C (M) | (−x) | +x | +2x | ||
| E (M) | — | x | 2x |
Substituting terms for the equilibrium concentrations into the solubility product expression and solving for x gives:
As defined in the ICE table, x is the molarity of calcium ion in the saturated solution. The dissolution stoichiometry shows a 1:1 relation between moles of calcium ion in solution and moles of compound dissolved, and so, the molar solubility of Ca(OH)2 is 6.9 × 10–3 M.
Check Your Learning
Click here for a walkthrough!
The dissolution equation and solubility product expression are:
The ICE table for this system is:
| PbI2 (s) | ⇌ | Pb2+(aq) | + | 2 I−(aq) | |
| I (M) | — | 0 | 0 | ||
| C (M) | (−x) | +x | +2x | ||
| E (M) | — | x | 2x |
Substituting terms for the equilibrium concentrations into the solubility product expression and solving for x gives:
As defined in the ICE table, x is the molarity of lead ion in the saturated solution. The dissolution stoichiometry shows a 1:1 relation between moles of lead ion in solution and moles of compound dissolved, so the molar solubility of PbI2 is 1.5 × 10–3 M.
Example 15.5 – Determination of Ksp from Gram Solubility
Many of the pigments used by artists in oil-based paints (Figure 15.3) are sparingly soluble in water. For example, the solubility of the artist’s pigment chrome yellow, PbCrO4, is 4.6 × 10–6 g/L.
Determine the solubility product for PbCrO4.

Solution
Before calculating the solubility product, the provided solubility must be converted to molar solubility:
The dissolution equation for this compound is:
The dissolution stoichiometry shows a 1:1 relation between the molar amounts of compound and its two ions, and so both [Pb2+] and [CrO42−] are equal to the molar solubility of PbCrO4:
Check Your Learning
Click here for a walkthrough!
Before calculating the solubility product, the provided solubility must be converted to molar solubility:
The dissolution equation for this compound is:
The dissolution stoichiometry shows a 1:1 relation between the molar amounts of compound and its two ions, and so both [Tl+] and [Cl–] are equal to the molar solubility of TlCl:
Example 15.6 – Calculating the Solubility of Hg2Cl2
Calomel, Hg2Cl2, is a compound composed of the diatomic ion of mercury(I), Hg22+, and chloride ions, Cl–. Although most mercury compounds are now known to be poisonous, eighteenth-century physicians used calomel as a medication. Their patients rarely suffered any mercury poisoning from the treatments because calomel has a very low solubility, as suggested by its very small Ksp:
Calculate the molar solubility of Hg2Cl2.
Solution
The dissolution stoichiometry shows a 1 : 1 relation between the amount of compound dissolved and the amount of mercury (I) ions, and so the molar solubility of Hg2Cl2 is equal to the concentration of Hg22+ ions.
Following the ICE approach results in:
| Hg2Cl2(s) | ⇌ | Hg22+(aq) | + | 2 Cl−(aq) | |
| I (M) | — | 0 | 0 | ||
| C (M) | (−x) | +x | +2x | ||
| E (M) | — | x | 2x |
Substituting the equilibrium concentration terms into the solubility product expression and solving for x gives:
The dissolution stoichiometry shows the molar solubility of Hg2Cl2 is equal to [Hg22+], or 6.5 × 10–7M.
Check Your Learning
Click here for a walkthrough!
Following the ICE approach results in:
| MgF2 (s) | ⇌ | Mg2+ (aq) | + | 2 F– (aq) | |
| I (M) | — | 0 | 0 | ||
| C (M) | (−x) | +x | +2x | ||
| E (M) | — | x | 2x |
Substituting the equilibrium concentration terms into the solubility product expression and solving for x gives:
The dissolution stoichiometry shows a 1:1 relation between the amount of compound dissolved and the amount of Mg2+ ions, and so the molar solubility of MgF2 is equal to 1.2 × 10–3 M.
How Sciences Interconnect
Using Barium Sulfate for Medical Imaging
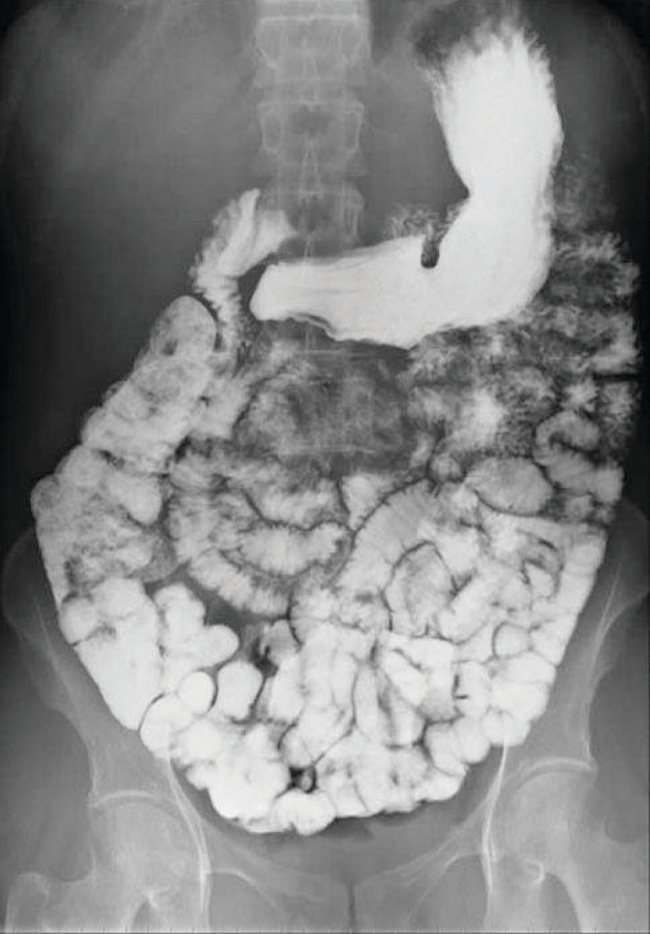
Various types of medical imaging techniques are used to aid diagnoses of illnesses in a noninvasive manner. One such technique utilizes the ingestion of a barium compound before taking an X-ray image. A suspension of barium sulfate, a chalky powder, is ingested by the patient. Since the Ksp of barium sulfate is 2.3 × 10–8, very little of it dissolves as it coats the lining of the patient’s intestinal tract. Barium-coated areas of the digestive tract then appear on an X-ray as white, allowing for greater visual detail than a traditional X-ray (Figure 15.4).
Medical imaging using barium sulfate can be used to diagnose acid reflux disease, Crohn’s disease, and ulcers in addition to other conditions.
Visit this website for more information on how barium is used in medical diagnoses and which conditions it is used to diagnose.
