Chapter 19 Transition Metals and Coordination Chemistry
Chapter 19 Transition Metals and Coordination Chemistry
We have daily contact with many transition metals. Iron occurs everywhere—from the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. Chromium is useful as a protective plating on plumbing fixtures and automotive detailing.
In addition to being used in their pure elemental forms, many compounds containing transition metals have numerous other applications. Silver nitrate is used to create mirrors, zirconium silicate provides friction in automotive brakes, and many important cancer-fighting agents, like the drug cisplatin and related species, are platinum compounds.
The variety of properties exhibited by transition metals is due to their complex valence shells. Unlike most main group metals where one oxidation state is normally observed, the valence shell structure of transition metals means that they usually occur in several different stable oxidation states. In addition, electron transitions in these elements can correspond with absorption of photons in the visible electromagnetic spectrum, leading to colored compounds. Because of these behaviors, transition metals exhibit a rich and fascinating chemistry.
Transition metals are especially useful in many aspects of chemistry that involve redox reactions as introduced in Chapter 17. Many transition metals can readily adopt a variety of oxidation states, which makes them particularly suited for use in important redox reactions in nature as well as the chemistry lab.
Photosystem II in plant photosynthesis includes a special catalyst called the oxygen evolving complex (OEC) that oxidizes water. This reaction requires oxidizing oxygen from an oxidation state of -2 to 0. Oxygen is famously reluctant to give up electrons, so this is truly an amazing feat of nature taking place all around us and allowing the creation of stored energy in the form of chemical bonds driven by sunlight! Our fossil fuels were produced over eons by storing such chemical energy in plant and subsequently animal biomass. So you could say that a lot of the energy we use to power our modern lives is a form of solar energy!
Of course, one drawback for using fossil fuels and other stored chemical energy to create power is the chemical byproducts of combustion – primarily carbon dioxide and water but also various sulfur and nitrogen compounds among others in the case of fossil fuel burning. Likewise, recall entropy and the second law of thermodynamics discussed in Chapter 16. This means producing power (work) from heat energy (q) is destined not to be very efficient and will result in a lot of wasted energy transforming heat to work in real world applications.
Photosystem II is able to oxidize water directly using sunlight to power the OEC catalyst which is comprised of four manganese transition metals along with calcium and oxygen. These manganese cycle through multiple oxidation states while driving the chemical reaction to form molecular oxygen from water and produce the electrons necessary for photosynthesis. Determining the specific oxidation states of each metal is a challenge. You can learn more about this in “Metal Oxidation States in Biological Water Splitting” by Krewald et al. in Chemical Science volume 6, pages 1676–95 published in 2015.
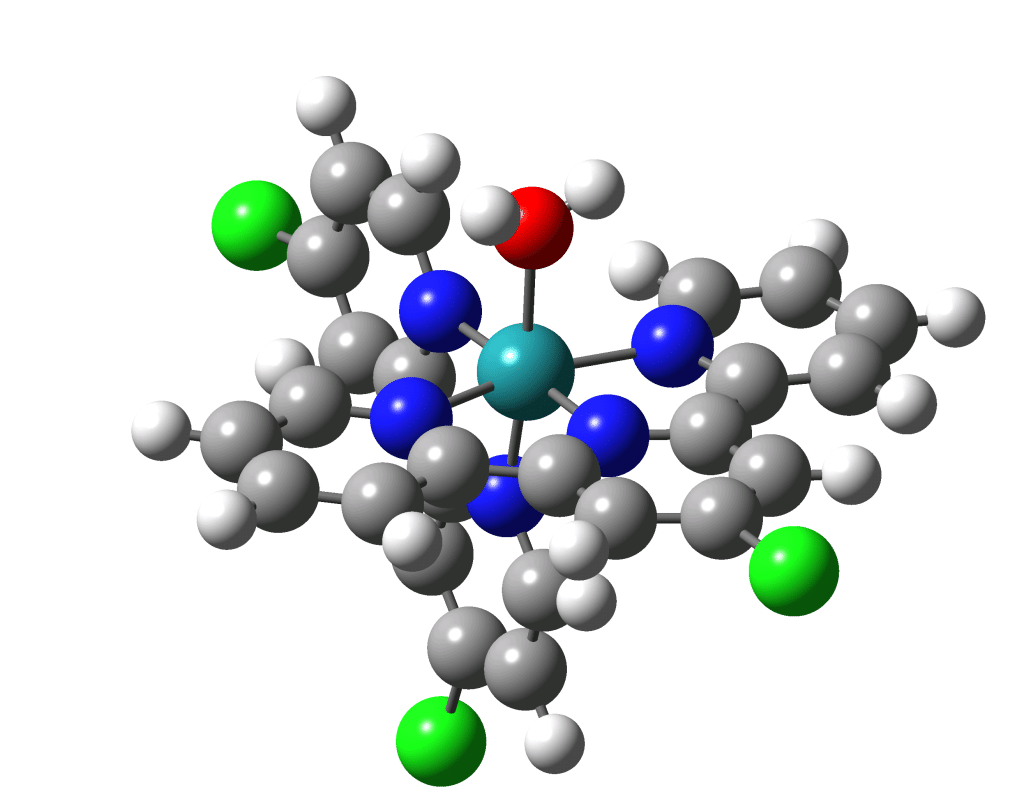
The water oxidation by the OEC serves as inspiration for many synthetic approaches to oxidizing water using catalysts containing transition metals—initially rare metals such as ruthenium and iridium but more recently expanding to more common transition metals as well such as the molecule shown in Figure 19.1. This is an active and rich area of study that hopefully will contribute to cleaner energy through solar production of hydrogen fuel and other technologies.
Media Attributions
- watoxCl3
