Chapter 2 Atoms, Molecules, and Ions
2.6 Ionic and Molecular Compounds
Learning Objectives
By the end of this section, you will be able to:
- Define ionic and molecular (covalent) compounds
- Predict the type of compound formed from elements based on their location within the periodic table
- Determine formulas for simple ionic compounds
In ordinary chemical reactions, the nucleus of each atom (and thus the identity of the element) remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (Figure 2.28).
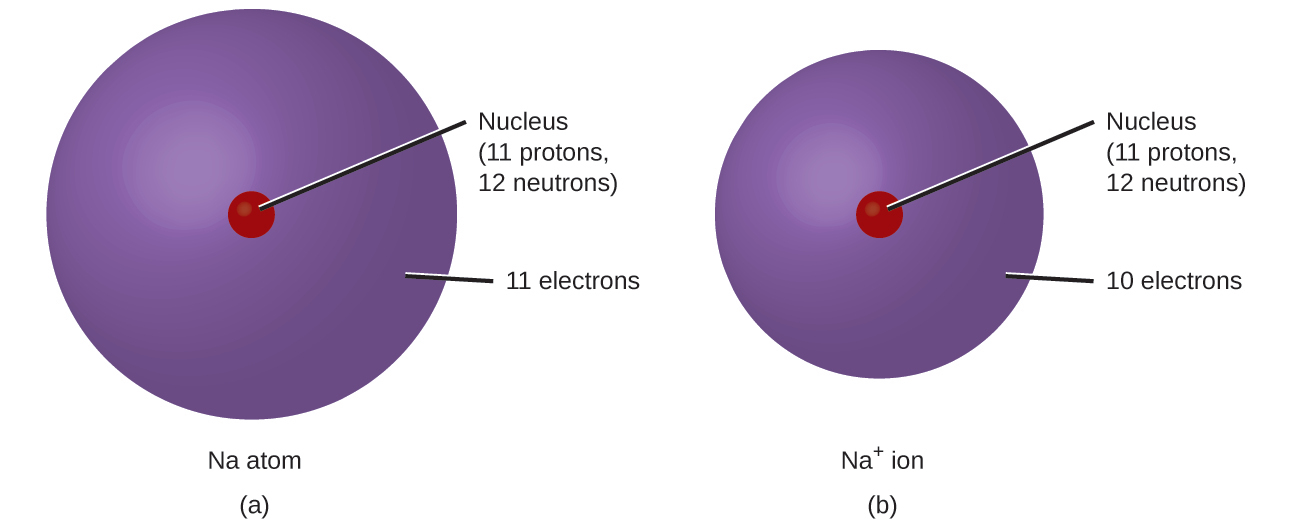
You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge, an alkaline earth metal (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. This results in a cation with 20 protons, 18 electrons, and a 2+ charge. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized Ca2+. The name of a metal ion is the same as the name of the metal atom from which it forms, so Ca2+ is called a calcium ion.
When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. Atoms of group 17 gain one electron and form anions with a 1− charge, atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. This results in an anion with 35 protons, 36 electrons, and a 1− charge. It has the same number of electrons as atoms of the next noble gas, krypton, and is symbolized Br−. (A discussion of the theory supporting the favored status of noble gas electron numbers reflected in these predictive rules for ion formation is provided in a later chapter of this text.)
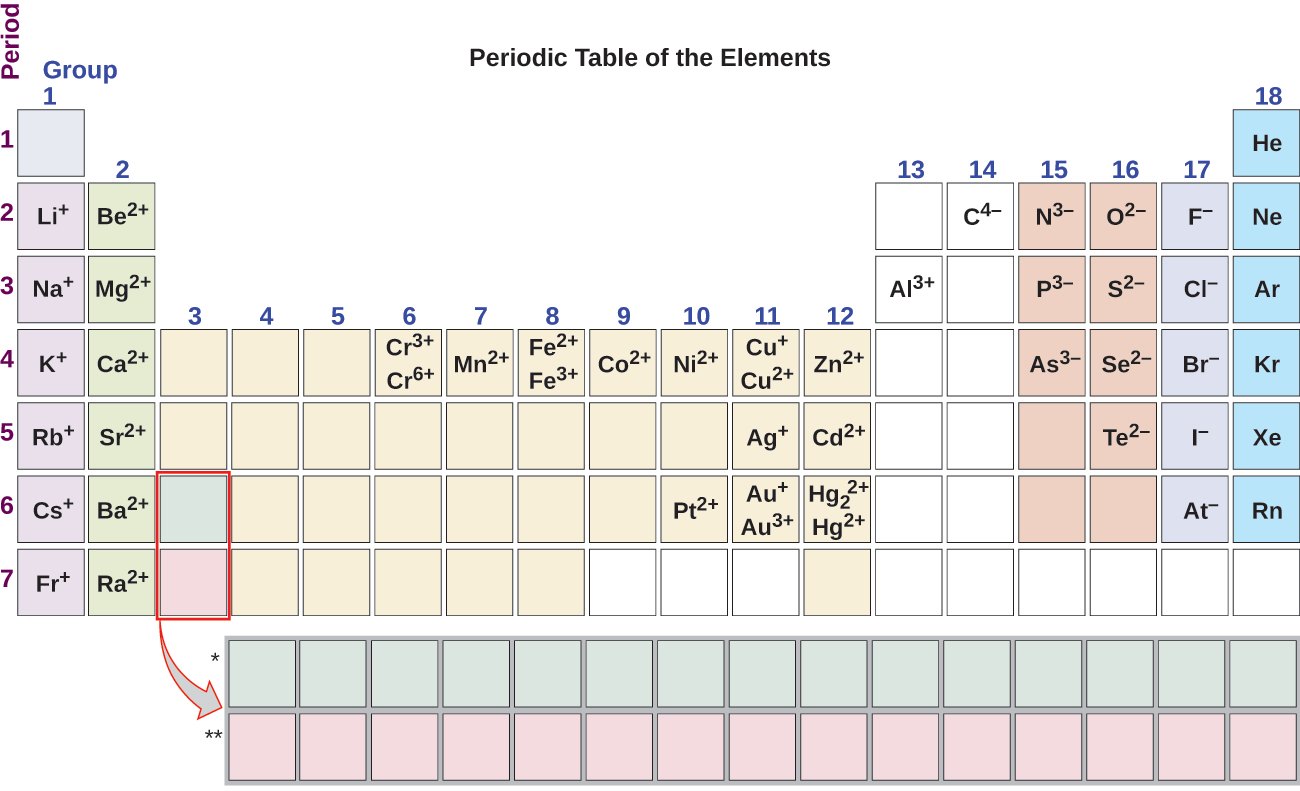
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure 2.29). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions, group 2 elements form 2+ ions, and so on. Moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. For example, group 17 elements (one group left of the noble gases) form 1− ions, group 16 elements (two groups left) form 2− ions, and so on. This trend can be used as a guide in many cases, but its predictive value decreases when moving toward the center of the periodic table. In fact, transition metals and some other metals often exhibit variable charges that are not predictable by their location in the table. For example, copper often forms ions with a 1+ or 2+ charge, and iron commonly form ions with a 2+ or 3+ charge.
Example 2.8 − Composition of Ions
An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. What is its symbol?
Solution
Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Knowing this lets us use the periodic table to identify the element as Al (aluminum). The Al atom has lost three electrons and thus has three more positive charges (13) than it has electrons (10). This is the aluminum cation, Al3+.
Check Your Learning
Example 2.9 − Formation of Ions
Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
Solution
Magnesium’s position in the periodic table (group 2) tells us that it is a metal. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number of electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Nonmetals form negative ions (anions). A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas, neon. Thus, a nitrogen atom will form an anion with three more electrons than protons and a charge of 3−. The symbol for the ion is N3−, and it is called a nitride ion.
Check Your Learning
Aluminum and carbon react to form an ionic compound.
Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and name them.
Click here for the answers!
- Al will form a cation with a charge of 3+: Al3+, an aluminum ion.
- Carbon will form an anion with a charge of 4−: C4-, a carbide ion
The ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one atom. We also find many polyatomic ions. These ions, which act as discrete units, are electrically charged molecules (a group of bonded atoms with an overall charge). Some of the more important polyatomic ions are listed in Table 2.5. Oxyanions are polyatomic ions that contain one or more oxygen atoms. At this point in your study of chemistry, you should memorize the names, formulas, and charges of the most common polyatomic ions. Because you will use them repeatedly, they will soon become familiar.
|
Name |
Formula |
Related Acid |
Formula |
|---|---|---|---|
|
ammonium |
NH4+ |
|
|
|
hydronium |
H3O+ |
||
|
peroxide |
O22− |
|
|
|
hydroxide |
OH− |
|
|
|
acetate |
CH3COO− |
acetic acid |
CH3COOH |
|
cyanide |
CN− |
hydrocyanic acid |
HCN |
|
azide |
N3− |
hydrazoic acid |
HN3 |
|
carbonate |
CO32− |
carbonic acid |
H2CO3 |
|
bicarbonate |
HCO3− |
|
|
|
nitrate |
NO3− |
nitric acid |
HNO3 |
|
nitrite |
NO2− |
nitrous acid |
HNO2 |
|
sulfate |
SO42− |
sulfuric acid |
H2SO4 |
|
hydrogen sulfate |
HSO4− |
|
|
|
sulfite |
SO32− |
sulfurous acid |
H2SO3 |
|
hydrogen sulfite |
HSO3− |
|
|
|
phosphate |
PO43− |
phosphoric acid |
H3PO4 |
|
hydrogen phosphate |
HPO42− |
|
|
|
dihydrogen phosphate |
H2PO4− |
|
|
|
perchlorate |
ClO4− |
perchloric acid |
HClO4 |
|
chlorate |
ClO3− |
chloric acid |
HClO3 |
|
chlorite |
ClO2− |
chlorous acid |
HClO2 |
|
hypochlorite |
ClO− |
hypochlorous acid |
HClO |
|
chromate |
CrO42− |
chromic acid |
H2CrO4 |
|
dichromate |
Cr2O72− |
dichromic acid |
H2Cr2O7 |
|
permanganate |
MnO4− |
permanganic acid |
HMnO4 |
Note that there is a system for naming some polyatomic ions; -ate and -ite are suffixes designating polyatomic ions containing more or fewer oxygen atoms. Per- (short for “hyper”) and hypo- (meaning “under”) are prefixes meaning more oxygen atoms than -ate and fewer oxygen atoms than -ite, respectively. For example, perchlorate is ClO4−, chlorate is ClO3−, chlorite is ClO2−, and hypochlorite is ClO−. Unfortunately, the number of oxygen atoms corresponding to a given suffix or prefix is not consistent; for example, nitrate is NO3− while sulfate is SO42−.
This will be covered in more detail in the next module on nomenclature.
The nature of the attractive forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding. When electrons are transferred and ions form, ionic bonds result. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this case, cations and anions). When electrons are “shared” and molecules form, covalent bonds result. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Compounds are classified as ionic or molecular (covalent) on the basis of the bonds present in them.
Ionic Compounds
When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily gain electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. The compound formed by this transfer is stabilized by the electrostatic attractions (ionic bonds) between the ions of opposite charge present in the compound. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas (group 17) accepts one electron to form a chloride anion, Cl−, the resulting compound, NaCl, is composed of sodium ions and chloride ions in the ratio of one Na+ ion for each Cl− ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form CaCl2, which is composed of Ca2+ and Cl− ions in the ratio of one Ca2+ ion to two Cl− ions.
A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. However, it is not always true (for example, aluminum chloride, AlCl3, is not ionic).
You can often recognize ionic compounds because of their properties. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. For example, sodium chloride melts at 801°C and boils at 1,413°C. (As a comparison, the molecular compound water melts at 0°C and boils at 100°C.) In solid form, an ionic compound is not electrically conductive because its ions are unable to flow (“electricity” is the flow of charged particles). When molten, however, it can conduct electricity because its ions are able to move freely through the liquid (Figure 2.30).
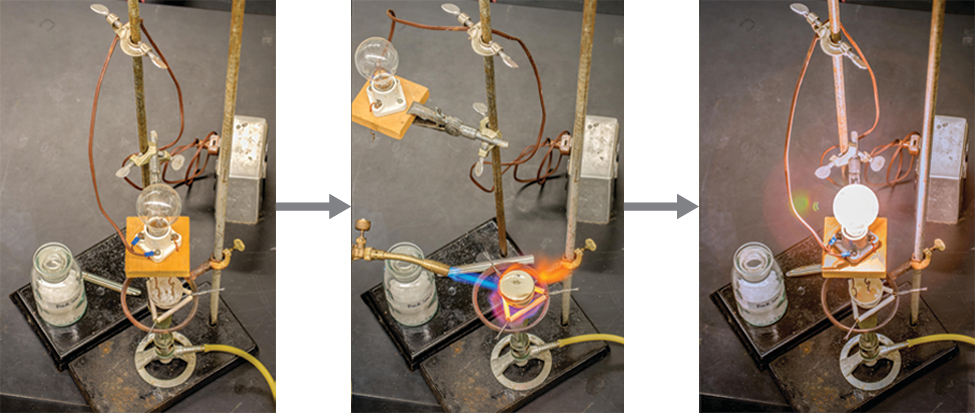
Link to Learning
Watch this video to see a mixture of salts melt and conduct electricity.
In every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. Thus, ionic compounds are electrically neutral overall, even though they contain positive and negative ions. We can use this observation to help us write the formula of an ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
Example 2.10 − Predicting the Formula of an Ionic Compound

The gemstone sapphire (Figure 2.31) is mostly a compound of aluminum and oxygen that contains aluminum cations, Al3+, and oxygen anions, O2−. What is the formula of this compound?
Solution
Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Two aluminum ions, each with a charge of 3+, would give us six positive charges, and three oxide ions, each with a charge of 2−, would give us six negative charges. The formula would be Al2O3.
Check Your Learning
Many ionic compounds contain polyatomic ions (Table 2.5) as the cation, the anion, or both. As with simple ionic compounds, these compounds must also be electrically neutral, so their formulas can be predicted by treating the polyatomic ions as discrete units. We use parentheses in a formula to indicate a group of atoms that behave as a unit. For example, the formula for calcium phosphate, one of the minerals in our bones, is Ca3(PO4)2. This formula indicates that there are three calcium ions (Ca2+) for every two phosphate (PO43−) groups. The PO43− groups are discrete units, each consisting of one phosphorus atom and four oxygen atoms, and having an overall charge of 3−. The compound is electrically neutral, and its formula shows a total count of three Ca, two P, and eight O atoms.
Example 2.11 − Predicting the Formula of a Compound with a Polyatomic Anion
Baking powder contains calcium dihydrogen phosphate, an ionic compound composed of the ions Ca2+ and H2PO4−. What is the formula of this compound?
Solution
The positive and negative charges must balance, and this ionic compound must be electrically neutral. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. This requires a ratio of one Ca2+ ion to two H2PO4−ions. We designate this by enclosing the formula for the dihydrogen phosphate ion in parentheses and adding a subscript 2. The formula is Ca(H2PO4)2.
Check Your Learning
Because an ionic compound is not made up of single, discrete molecules, it may not be properly symbolized using a molecular formula. Instead, ionic compounds must be symbolized by a formula indicating the relative numbers of its constituent ions. For compounds containing only monatomic ions (such as NaCl) and for many compounds containing polyatomic ions (such as CaSO4), these formulas are just the empirical formulas introduced earlier in this chapter. However, the formulas for some ionic compounds containing polyatomic ions are not empirical formulas. For example, the ionic compound sodium oxalate is comprised of Na+ and C2O42− ions combined in a 2:1 ratio, and its formula is written as Na2C2O4. The subscripts in this formula are not the smallest-possible whole numbers, as each can be divided by 2 to yield the empirical formula, NaCO2. This is not the accepted formula for sodium oxalate, however, as it does not accurately represent the compound’s polyatomic anion, C2O42−.
Molecular Compounds
Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Covalent bonding is an important and extensive concept in chemistry, and it will be treated in considerable detail in a later chapter of this text. We can often identify molecular compounds on the basis of their physical properties. Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist.
Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Thus, the periodic table can help us recognize many of the compounds that are covalent. While we often can use the positions of a compound’s elements in the periodic table to predict whether it is ionic or covalent, this is a simplistic approach that does not account for a number of interesting exceptions. Shades of gray exist between ionic and molecular compounds, and you’ll learn more about those later.
Example 2.12 − Predicting the Type of Bonding in Compounds
Predict whether the following compounds are ionic or molecular:
(a) KI, the compound used as a source of iodine in table salt
(b) H2O2, the bleach and disinfectant hydrogen peroxide
(c) CHCl3, the anesthetic chloroform
(d) Li2CO3, a source of lithium in antidepressants
Solution
(a) Potassium (group 1) is a metal, and iodine (group 17) is a nonmetal; KI is predicted to be ionic.
(b) Hydrogen (group 1) is a nonmetal, and oxygen (group 16) is a nonmetal; H2O2 is predicted to be molecular.
(c) Carbon (group 14) is a nonmetal, hydrogen (group 1) is a nonmetal, and chlorine (group 17) is a nonmetal; CHCl3 is predicted to be molecular.
(d) Lithium (group 1) is a metal, and carbonate is a polyatomic ion; Li2CO3 is predicted to be ionic.
Check Your Learning
