Chapter 2 Atoms, Molecules, and Ions
2.7 Chemical Nomenclature
Learning Objectives
By the end of this section, you will be able to:
- Derive names for common types of inorganic compounds using a systematic approach
Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). We will limit our attention here to inorganic compounds—compounds that are composed principally of elements other than carbon—and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principal element, will be treated in a later chapter on organic chemistry.
Ionic Compounds
To name an inorganic compound, we need to consider the answers to several questions:
- Is the compound ionic or molecular?
- If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)?
- Are the ions monatomic or polyatomic?
- If the compound is molecular, does it contain hydrogen? If so, does it also contain oxygen?
From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
Compounds Containing Only Monatomic Ions
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix –ide). Some examples are given in Table 2.6.
|
Formula |
Name |
|
Formula |
Name |
|---|---|---|---|---|
|
NaCl |
sodium chloride |
Na2O |
sodium oxide |
|
|
KBr |
potassium bromide |
CdS |
cadmium sulfide |
|
|
CaI2 |
calcium iodide |
Mg3N2 |
magnesium nitride |
|
|
CsF |
cesium fluoride |
Ca3P2 |
calcium phosphide |
|
|
LiCl |
lithium chloride |
Al4C3 |
aluminum carbide |
Compounds Containing Polyatomic Ions
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions (i.e., by naming first the cation and then the anion). Examples are shown in Table 2.7.
|
Formula |
Name |
|
Formula |
Name |
|---|---|---|---|---|
|
KC2H3O2 |
potassium acetate |
NH4Cl |
ammonium chloride |
|
|
NaHCO3 |
sodium bicarbonate |
CaSO4 |
calcium sulfate |
|
|
Al2(CO3)3 |
aluminum carbonate |
Mg3(PO4)2 |
magnesium phosphate |
Chemistry in Everyday Life
Ionic Compounds in Your Cabinets
Every day you encounter and use a large number of ionic compounds. Some of these compounds, where they are found, and what they are used for are listed in Table 2.8. Look at the label or ingredients list on the various products that you use during the next few days and see if you run into any of those in this table or find other ionic compounds that you could now name or write as a formula.
| Formula | Name | Use |
|---|---|---|
|
NaCl |
sodium chloride |
ordinary table salt |
|
KI |
potassium iodide |
added to “iodized” salt for thyroid health |
|
NaF |
sodium fluoride |
ingredient in toothpaste |
|
NaHCO3 |
sodium bicarbonate |
baking soda; used in cooking (and as antacid) |
|
Na2CO3 |
sodium carbonate |
washing soda; used in cleaning agents |
|
NaOCl |
sodium hypochlorite |
active ingredient in household bleach |
|
CaCO3 |
calcium carbonate |
ingredient in antacids |
|
Mg(OH)2 |
magnesium hydroxide |
ingredient in antacids |
|
Al(OH)3 |
aluminum hydroxide |
ingredient in antacids |
|
NaOH |
sodium hydroxide |
lye; used as drain cleaner |
|
K3PO4 |
potassium phosphate |
food additive (many purposes) |
|
MgSO4 |
magnesium sulfate |
added to purified water |
|
Na2HPO4 |
sodium hydrogen phosphate |
anti-caking agent; used in powdered products |
|
Na2SO3 |
sodium sulfite |
preservative |
Compounds Containing a Metal Ion with a Variable Charge
Most of the transition metals and some main group metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ (Figure 2.29), and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, “iron chloride,” will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron (II) chloride and iron (III) chloride, respectively. Other examples are provided in Table 2.9.
|
Compound |
Name |
|---|---|
|
FeCl2 |
iron (II) chloride |
|
FeCl3 |
iron (III) chloride |
|
Hg2O |
mercury (I) oxide |
|
HgO |
mercury (II) oxide |
|
SnF2 |
tin (II) fluoride |
|
SnF4 |
tin (IV) fluoride |
Out-of-date nomenclature used the suffixes –ic and –ous to designate metals with higher and lower charges, respectively: Iron (III) chloride, FeCl3, was previously called ferric chloride, and iron (II) chloride, FeCl2, was known as ferrous chloride. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula SnF2, which is more properly named tin (II) fluoride. The other fluoride of tin is SnF4, which was previously called stannic fluoride but is now named tin (IV) fluoride.
Ionic Hydrates
Ionic compounds that contain water molecules as integral components of their crystals are called hydrates. The name for an ionic hydrate is derived by adding a term to the name for the anhydrous (meaning “not hydrated”) compound that indicates the number of water molecules associated with each formula unit of the compound. The added word begins with a Greek prefix denoting the number of water molecules (Table 2.10) and ends with “hydrate.” For example, the anhydrous compound copper(II) sulfate also exists as a hydrate containing five water molecules and named copper(II) sulfate pentahydrate. Washing soda is the common name for a hydrate of sodium carbonate containing 10 water molecules; the systematic name is sodium carbonate decahydrate.
Formulas for ionic hydrates are written by appending a vertically centered dot, a coefficient representing the number of water molecules, and the formula for water. The two examples mentioned in the previous paragraph are represented by the following formulas:
copper(II) sulfate pentahydrate: CuSO4 • 5H2O
sodium carbonate decahydrate: Na2CO3 • 10H2O
|
Number |
Prefix |
Number |
Prefix |
|
|---|---|---|---|---|
|
1 (sometimes omitted)
|
mono- |
6 |
hexa- |
|
|
2 |
di- |
7 |
hepta- |
|
|
3 |
tri- |
8 |
octa- |
|
|
4 |
tetra- |
9 |
nona- |
|
|
5 |
penta- |
10 |
deca- |
Example 2.13 − Naming Ionic Compounds
Name the following ionic compounds:
(a) Fe2S3
(b) CuSe
(c) GaN
(d) MgSO4·7H2O
(e) Ti2(SO4)3
Solution
The anions in these compounds have fixed negative charges (S2−, Se2− , N3−, and SO42-), and the compounds must be neutral. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Mg2+, and Ti3+. These charges are used in the names of the metal ions:
(a) iron (III) sulfide
(b) copper (II) selenide
(c) gallium (III) nitride
(d) magnesium sulfate heptahydrate
(e) titanium (III) sulfate
Check Your Learning
Chemistry in Everyday Life
Erin Brockovich and Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich (Figure 2.32) discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr(VI) used by Pacific Gas & Electric (PG&E) to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brockovich (for which Julia Roberts won an Oscar), Erin and lawyer Edward Masry sued PG&E for contaminating the water near Hinckley in 1993. The settlement they won in 1996—$333 million—was the largest amount ever awarded for a direct-action lawsuit in the United States at that time.
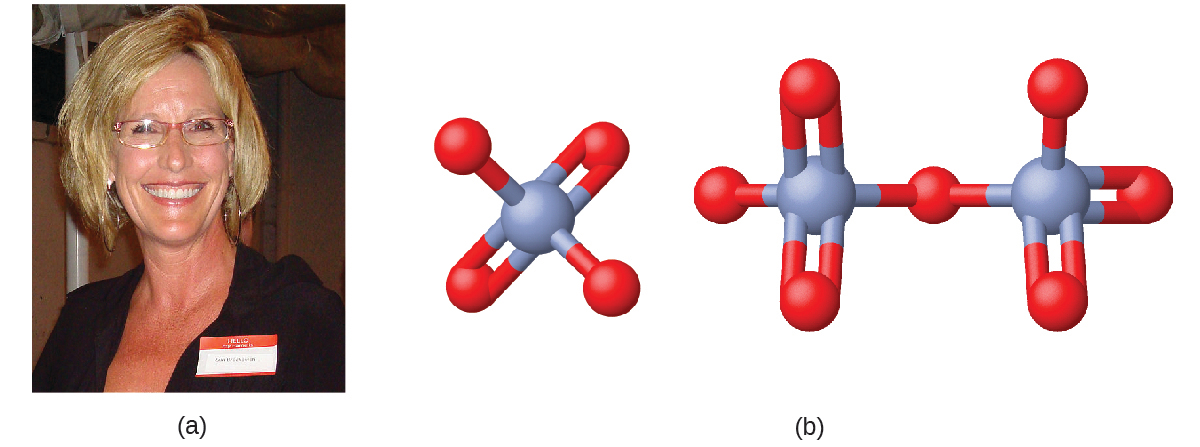
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr (III) or Cr (VI) forms. Cr (III), an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr (VI) is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr (VI) can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin. Despite cleanup efforts, Cr (VI) groundwater contamination remains a problem in Hinckley and other locations across the globe. A 2010 study by the Environmental Working Group found that of 35 US cities tested, 31 had higher levels of Cr (VI) in their tap water than the public health goal of 0.02 parts per billion set by the California Environmental Protection Agency.
Molecular (Covalent) Compounds
The bonding characteristics of inorganic molecular compounds are different from ionic compounds, and they are named using a different system as well. The charges of cations and anions dictate their ratios in ionic compounds, so specifying the names of the ions provides sufficient information to determine chemical formulas. However, because covalent bonding allows for significant variation in the combination ratios of the atoms in a molecule, the names for molecular compounds must explicitly identify these ratios.
Compounds Composed of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO2. Since these are different substances with different properties, they cannot both have the same name (they cannot both be called carbon oxide). To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element (the one farther to the left and/or bottom of the periodic table) is first, followed by the name of the more nonmetallic element (the one farther to the right and/or top) with its ending changed to the suffix –ide. The numbers of atoms of each element are designated by the Greek prefixes shown in Table 2.10.
When only one atom of the first element is present, the prefix mono– is usually deleted from that part. Thus, CO is named carbon monoxide, and CO2 is called carbon dioxide. When two vowels are adjacent, the a in the Greek prefix is usually dropped. Some other examples are shown in Table 2.11.
|
Compound |
Name |
|
Compound |
Name |
|---|---|---|---|---|
|
SO2 |
sulfur dioxide |
BCl3 |
boron trichloride |
|
|
SO3 |
sulfur trioxide |
SF6 |
sulfur hexafluoride |
|
|
NO2 |
nitrogen dioxide |
PF5 |
phosphorus pentafluoride |
|
|
N2O4 |
dinitrogen tetroxide |
P4O10 |
tetraphosphorus decaoxide |
|
|
N2O5 |
dinitrogen pentoxide |
IF7 |
iodine heptafluoride |
There are a few common names that you will encounter as you continue your study of chemistry. For example, although NO is often called nitric oxide, its proper name is nitrogen monoxide. Similarly, N2O is known as nitrous oxide even though our rules would specify the name dinitrogen monoxide. (And H2O is usually called water, not dihydrogen monoxide.) You should commit to memory the common names of compounds as you encounter them.
Example 2.14 − Naming Covalent Compounds
Name the following covalent compounds:
(a) SF6
(b) N2O3
(c) Cl2O7
(d) P4O6
Solution
Because these compounds consist solely of nonmetals, we use prefixes to designate the number of atoms of each element:
(a) sulfur hexafluoride
(b) dinitrogen trioxide
(c) dichlorine heptoxide
(d) tetraphosphorus hexoxide
Check Your Learning
Link to Learning
The following website provides practice with naming chemical compounds and writing chemical formulas. You can choose binary, polyatomic, and variable-charge ionic compounds, as well as molecular compounds.
Binary Acids
Some compounds containing hydrogen are members of an important class of substances known as acids. The chemistry of these compounds is explored in more detail in later chapters of this text, but for now, it will suffice to note that many acids release hydrogen ions, H+, when dissolved in water. To denote this distinct chemical property, a mixture of water with an acid is given a name derived from the compound’s name. If the compound is a binary acid (comprised of hydrogen and one other nonmetallic element), the compound’s name follows these rules:
- The word “hydrogen” is changed to the prefix hydro-.
- The other nonmetallic element name is modified by adding the suffix –ic.
- The word “acid” is added as a second word.
For example, when the gas HCl (hydrogen chloride) is dissolved in water, the solution is called hydrochloric acid. Several other examples of this nomenclature are shown in Table 2.12.
|
Compound |
Name of Gas |
Name of Acid |
|---|---|---|
|
HF |
hydrogen fluoride |
hydrofluoric acid |
|
HCl |
hydrogen chloride |
hydrochloric acid |
|
HBr |
hydrogen bromide |
hydrobromic acid |
|
HI |
hydrogen iodide |
hydroiodic acid |
|
H2S |
hydrogen sulfide |
hydrosulfuric acid |
Oxyacids
Many compounds containing three or more elements (such as organic compounds or coordination compounds) are subject to specialized nomenclature rules that you will learn later. However, we will briefly discuss the important compounds known as oxyacids, compounds that contain hydrogen, oxygen, and at least one other element, and are bonded in such a way as to impart acidic properties to the compound (you will learn the details of this in a later chapter). Typical oxyacids consist of hydrogen combined with a polyatomic, oxygen-containing ion. To name oxyacids, follow these rules:
- Omit “hydrogen.”
- Start with the root name of the anion.
- Replace –ate with –ic or –ite with –ous.
- Add “acid.”
For example, consider H2CO3 (which you might be tempted to call “hydrogen carbonate”). To name this correctly, “hydrogen” is omitted; the –ate of carbonate is replace with –ic; and acid is added—so its name is carbonic acid. Other examples are given in Table 2.13. There are some exceptions to the general naming method (e.g., H2SO4 is called sulfuric acid, not sulfic acid, and H2SO3 is sulfurous acid, not sulfous acid).
|
Formula |
Anion Name |
Acid Name |
|---|---|---|
|
HC2H3O2 |
acetate |
acetic acid |
|
HNO3 |
nitrate |
nitric acid |
|
HNO2 |
nitrite |
nitrous acid |
|
HClO4 |
perchlorate |
perchloric acid |
|
H2CO3 |
carbonate |
carbonic acid |
|
H2SO4 |
sulfate |
sulfuric acid |
|
H2SO3 |
sulfite |
sulfurous acid |
|
H3PO4 |
phosphate |
phosphoric acid |
