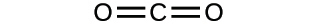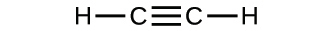Chapter 2 Atoms, Molecules, and Ions
Chapter 2 Problems
2.1 Early Ideas in Atomic Theory
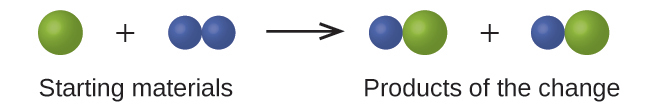
2.1. In the following drawing, the green spheres represent atoms of a certain element. The purple spheres represent atoms of another element. If the spheres of different elements touch, they are part of a single unit of a compound. The following chemical change represented by these spheres may violate one of the ideas of Dalton’s atomic theory. Which one?
2.2. Which postulate of Dalton’s theory is consistent with the following observation concerning the weights of reactants and products? When 100 grams of solid calcium carbonate is heated, 44 grams of carbon dioxide and 56 grams of calcium oxide are produced.
2.3. Identify the postulate of Dalton’s theory that is violated by the following observations: 59.95% of one sample of titanium dioxide is titanium; 60.10% of a different sample of titanium dioxide is titanium.
2.4. Samples of compound X, Y, and Z are analyzed, with results shown here.
|
Compound |
Description |
Mass of Carbon |
Mass of Hydrogen |
|---|---|---|---|
|
X |
clear, colorless, liquid with strong odor |
1.776 g |
0.148 g |
|
Y |
clear, colorless, liquid with strong odor |
1.974 g |
0.329 g |
|
Z |
clear, colorless, liquid with strong odor |
7.812 g |
0.651 g |
Do these data provide example(s) of the law of definite proportions, the law of multiple proportions, neither, or both? What do these data tell you about compounds X, Y, and Z?
2.2 Evolution of Atomic Theory
2.5. The existence of isotopes violates one of the original ideas of Dalton’s atomic theory. Which one?
2.6. How are electrons and protons similar? How are they different?
2.7. How are protons and neutrons similar? How are they different?
2.8. Predict and test the behavior of α particles fired at a “plum pudding” model atom.
(a) Predict the paths taken by α particles that are fired at atoms with a Thomson’s plum pudding model structure. Explain why you expect the α particles to take these paths.
(b) If α particles of higher energy than those in (a) are fired at plum pudding atoms, predict how their paths will differ from the lower-energy α particle paths. Explain your reasoning.
(c) Now test your predictions from (a) and (b). Open the Rutherford Scattering simulation and select the “Plum Pudding Atom” tab. Set “Alpha Particles Energy” to “min,” and select “show traces.” Click on the gun to start firing α particles. Does this match your prediction from (a)? If not, explain why the actual path would be that shown in the simulation. Hit the pause button, or “Reset All.” Set “Alpha Particles Energy” to “max,” and start firing α particles. Does this match your prediction from (b)? If not, explain the effect of increased energy on the actual paths as shown in the simulation.
2.9. Predict and test the behavior of α particles fired at a Rutherford atom model.
(a) Predict the paths taken by α particles that are fired at atoms with a Rutherford atom model structure. Explain why you expect the α particles to take these paths.
(b) If α particles of higher energy than those in (a) are fired at Rutherford atoms, predict how their paths will differ from the lower-energy α particle paths. Explain your reasoning.
(c) Predict how the paths taken by the α particles will differ if they are fired at Rutherford atoms of elements other than gold. What factor do you expect to cause this difference in paths, and why?
(d) Now test your predictions from (a), (b), and (c). Open the Rutherford Scattering simulation and select the “Rutherford Atom” tab. Due to the scale of the simulation, it is best to start with a small nucleus, so select “20” for both protons and neutrons, “min” for energy, show traces, and then start firing α particles. Does this match your prediction from (a)? If not, explain why the actual path would be that shown in the simulation. Pause or reset, set energy to “max,” and start firing α particles. Does this match your prediction from (b)? If not, explain the effect of increased energy on the actual path as shown in the simulation. Pause or reset, select “40” for both protons and neutrons, “min” for energy, show traces, and fire away. Does this match your prediction from (c)? If not, explain why the actual path would be that shown in the simulation. Repeat this with larger numbers of protons and neutrons. What generalization can you make regarding the type of atom and effect on the path of α particles? Be clear and specific.
2.3 Atomic Structure and Symbolism
2.10. In what way are isotopes of a given element always different? In what way(s) are they always the same?
2.11. Write the symbol for each of the following ions:
(a) the ion with a 1+ charge, atomic number 55, and mass number 133
(b) the ion with 54 electrons, 53 protons, and 74 neutrons
(c) the ion with atomic number 15, mass number 31, and a 3− charge
(d) the ion with 24 electrons, 30 neutrons, and a 3+ charge
2.12. Write the symbol for each of the following ions:
(a) the ion with a 3+ charge, 28 electrons, and a mass number of 71
(b) the ion with 36 electrons, 35 protons, and 45 neutrons
(c) the ion with 86 electrons, 142 neutrons, and a 4+ charge
(d) the ion with a 2+ charge, atomic number 38, and mass number 87
2.13. Open the Build an Atom simulation and click on the Atom icon.
(a) Pick any one of the first 10 elements that you would like to build and state its symbol.
(b) Drag protons, neutrons, and electrons onto the atom template to make an atom of your element. State the numbers of protons, neutrons, and electrons in your atom, as well as the net charge and mass number.
(c) Click on “Net Charge” and “Mass Number,” check your answers to (b), and correct, if needed.
(d) Predict whether your atom will be stable or unstable. State your reasoning.
(e) Check the “Stable/Unstable” box. Was your answer to (d) correct? If not, first predict what you can do to make a stable atom of your element, and then do it and see if it works. Explain your reasoning.
2.14. Open the Build an Atom simulation.
(a) Drag protons, neutrons, and electrons onto the atom template to make a neutral atom of Oxygen-16 and give the isotope symbol for this atom.
(b) Now add two more electrons to make an ion and give the symbol for the ion you have created.
2.15. Open the Build an Atom simulation.
(a) Drag protons, neutrons, and electrons onto the atom template to make a neutral atom of Lithium-6 and give the isotope symbol for this atom.
(b) Now remove one electron to make an ion and give the symbol for the ion you have created.
2.16. Determine the number of protons, neutrons, and electrons in the following isotopes that are used in medical diagnoses:
(a) atomic number 9, mass number 18, charge of 1−
(b) atomic number 43, mass number 99, charge of 7+
(c) atomic number 53, atomic mass number 131, charge of 1−
(d) atomic number 81, atomic mass number 201, charge of 1+
(e) Name the elements in parts (a), (b), (c), and (d).
2.17. The following are properties of isotopes of two elements that are essential in our diet. Determine the number of protons, neutrons, and electrons in each and name them.
2.18. Give the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes:
(a)
(b)
(c)
(d)
(e)
2.19. Give the number of protons, electrons, and neutrons in neutral atoms of each of the following isotopes:
(a)
(b)
(c)
(d)
(e)
2.20. Click the link to this site and select the “Mix Isotopes” tab, hide the “Percent Composition” and “Average Atomic Mass” boxes, and then select the element boron.
(a) Write the symbols of the isotopes of boron that are shown as naturally occurring in significant amounts.
(b) Predict the relative amounts (percentages) of these boron isotopes found in nature. Explain the reasoning behind your choice.
(c) Add isotopes to the black box to make a mixture that matches your prediction in (b). You may drag isotopes from their bins or click on “More” and then move the sliders to the appropriate amounts.
(d) Reveal the “Percent Composition” and “Average Atomic Mass” boxes. How well does your mixture match with your prediction? If necessary, adjust the isotope amounts to match your prediction.
(e) Select “Nature’s” mix of isotopes and compare it to your prediction. How well does your prediction compare with the naturally occurring mixture? Explain. If necessary, adjust your amounts to make them match “Nature’s” amounts as closely as possible.
2.21. Repeat Problem 2.20 using an element that has three naturally occurring isotopes.
2.22. An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99 amu, 0.26% abundance with 20.99 amu, and 8.82% abundance with 21.99 amu. Calculate the average atomic mass of this element.
2.23.
2.24. Variations in average atomic mass may be observed for elements obtained from different sources. Lithium provides an example of this. The isotopic composition of lithium from naturally occurring minerals is 7.5% 6Li and 92.5% 7Li, which have masses of 6.01512 amu and 7.01600 amu, respectively. A commercial source of lithium, recycled from a military source, was 3.75% 6Li (and the rest 7Li). Calculate the average atomic mass values for each of these two sources.
2.25. The average atomic masses of some elements may vary, depending upon the sources of their ores. Naturally occurring boron consists of two isotopes with accurately known masses (10B, 10.0129 amu and 11B, 11.00931 amu). The actual atomic mass of boron can vary from 10.807 to 10.819, depending on whether the mineral source is from Turkey or the United States.
2.26. The 18O:16O abundance ratio in some meteorites is greater than that used to calculate the average atomic mass of oxygen on earth. Is the average mass of an oxygen atom in these meteorites greater than, less than, or equal to that of a terrestrial oxygen atom?
2.4 Chemical Formulas
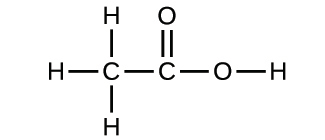
2.27. Explain why the symbol for an atom of the element oxygen and the formula for a molecule of oxygen differ.
2.28. Explain why the symbol for the element sulfur and the formula for a molecule of sulfur differ.
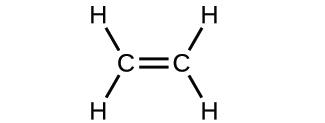
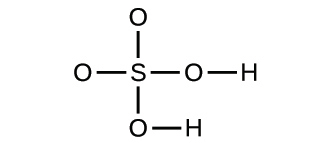
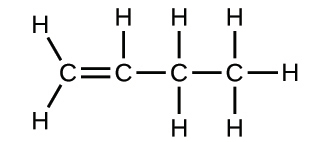
2.29. Write the molecular and empirical formulas of the following compounds:
(a)
(b)
(c)
(d)
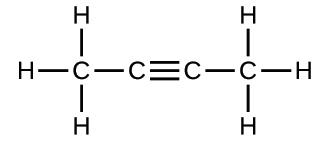
2.30. Write the molecular and empirical formulas of the following compounds:
(a)
(b)
(c)
(d)
2.31. Determine the empirical formulas for the following compounds:
(a) caffeine, C8H10N4O2
(b) sucrose, C12H22O11
(c) hydrogen peroxide, H2O2
(d) glucose, C6H12O6
(e) ascorbic acid (vitamin C), C6H8O6
2.32. Determine the empirical formulas for the following compounds:
(a) acetic acid, C2H4O2
(b) citric acid, C6H8O7
(c) hydrazine, N2H4
(d) nicotine, C10H14N2
(e) butane, C4H10
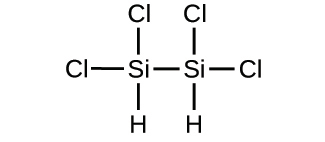
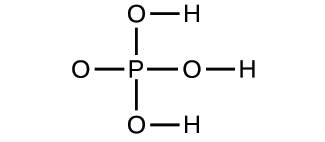
2.33. Write the empirical formulas for the following compounds:
(a)
(b)
2.34. Open the Build a Molecule simulation and select the “Larger Molecules” tab. Select an appropriate atom’s “Kit” to build a molecule with two carbon and six hydrogen atoms. Drag atoms into the space above the “Kit” to make a molecule. A name will appear when you have made an actual molecule that exists (even if it is not the one you want). You can use the scissors tool to separate atoms if you would like to change the connections. Click on “3D” to see the molecule, and look at both the space-filling and ball-and-stick possibilities.
(a) Draw the structural formula of this molecule and state its name.
(b) Can you arrange these atoms in any way to make a different compound?
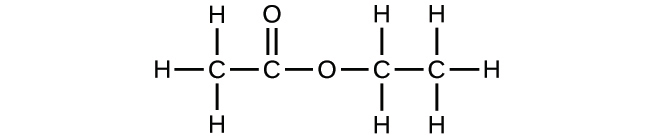
2.35. Use the Build a Molecule simulation to repeat Problem 2.34, but build a molecule with two carbons, six hydrogens, and one oxygen.
(a) Draw the structural formula of this molecule and state its name.
(b) Can you arrange these atoms to make a different molecule? If so, draw its structural formula and state its name.
(c) How are the molecules drawn in (a) and (b) the same? How do they differ? What are they called (the type of relationship between these molecules, not their names).?
2.36. Use the Build a Molecule simulation to repeat Problem 2.34, but build a molecule with three carbons, seven hydrogens, and one chlorine.
(a) Draw the structural formula of this molecule and state its name.
(b) Can you arrange these atoms to make a different molecule? If so, draw its structural formula and state its name.
(c) How are the molecules drawn in (a) and (b) the same? How do they differ? What are they called (the type of relationship between these molecules, not their names)?
2.5 The Periodic Table
2.37. Using the periodic table, classify each of the following elements as: (i) a metal or a nonmetal and (ii) a main-group (representative) element, a transition metal, or an inner-transition metal by clicking on the key word.
2.38. Using the periodic table, classify each of the following elements as a metal or a nonmetal, and then further classify each as a main-group (representative) element, transition metal, or inner transition metal:
(a) cobalt
(b) europium
(c) iodine
(d) indium
(e) lithium
(f) oxygen
(g) cadmium
(h) terbium
(i) rhenium
2.40. Using the periodic table, identify the heaviest member of each of the following groups:
(a) alkali metals
(b) chalcogens
(c) noble gases
(d) alkaline earth metals
2.41.
2.42. Use the periodic table to give the name and symbol for each of the following elements:
(a) the halogen in the same period as the alkali metal with 11 protons
(b) the alkaline earth metal in the same period with the neutral noble gas with 18 electrons
(c) the noble gas in the same row as an isotope with 30 neutrons and 25 protons
(d) the noble gas in the same period as gold
2.43. Write a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.
(a) the alkali metal with 11 protons and a mass number of 23
(b) the noble gas element with 75 neutrons in its nucleus and 54 electrons in the neutral atom
(c) the isotope with 33 protons and 40 neutrons in its nucleus
(d) the alkaline earth metal with 88 electrons and 138 neutrons
2.44. Write a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.
(a) the chalcogen with a mass number of 125
(b) the halogen whose longest-lived isotope is radioactive
(c) the noble gas, used in lighting, with 10 electrons and 10 neutrons
(d) the lightest alkali metal with three neutrons
2.6 Ionic and Molecular Compounds
2.45. Using the periodic table, predict whether the following chlorides are ionic or covalent
2.46. Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl4, PCl3, CaCl2, CsCl, CuCl2, and CrCl3.
2.47. For each of the following compounds, state whether it is ionic or covalent. If it is ionic, write the symbols for the ions involved:
(a) NF3
(b) BaO
(c) (NH4)2CO3
(d) Sr(H2PO4)2
(e) IBr
(f) Na2O
2.48. For each of the following compounds, state whether it is ionic or covalent, and if it is ionic, write the symbols for the ions involved:
(a) KClO4
(b) Mg(C2H3O2)2
(c) H2S
(d) Ag2S
(e) N2Cl4
(f) Co(NO3)2
2.49. For each of the following pairs of ions, write the formula of the compound they will form:
(a) Ca2+, S2−
(b) NH4+, SO42−
(c) Al3+, Br−
(d) Na+, HPO42−
(e) Mg2+, PO43−
2.50. For each of the following pairs of ions, write the formula of the compound they will form:
(a) K+, O2−
(b) NH4+, PO43−
(c) Al3+, O2−
(d) Na+, CO32−
(e) Ba2+, PO43−
2.7 Chemical Nomenclature
2.51.
2.52. Name the following compounds:
(a) NaF
(b) Rb2O
(c) BCl3
(d) H2Se
(e) P4O6
(f) ICl3
2.53. Write the formulas of the following compounds:
(a) rubidium bromide
(b) magnesium selenide
(c) sodium oxide
(d) calcium chloride
(e) hydrogen fluoride
(f) gallium phosphide
(g) aluminum bromide
(h) ammonium sulfate
2.54. Write the formulas of the following compounds:
(a) lithium carbonate
(b) sodium perchlorate
(c) barium hydroxide
(d) ammonium carbonate
(e) sulfuric acid
(f) calcium acetate
(g) magnesium phosphate
(h) sodium sulfite
2.55. Write the formulas of the following compounds:
(a) chlorine dioxide
(b) dinitrogen tetraoxide
(c) potassium phosphide
(d) silver (I) sulfide
(e) aluminum fluoride trihydrate
(f) silicon dioxide
2.56. Write the formulas of the following compounds:
(a) barium chloride
(b) magnesium nitride
(c) sulfur dioxide
(d) nitrogen trichloride
(e) dinitrogen trioxide
(f) tin (IV) chloride
2.57. Each of the following compounds contains a metal that can exhibit more than one ionic charge. Name these compounds:
(a) Cr2O3
(b) FeCl2
(c) CrO3
(d) TiCl4
(e) CoCl2·6H2O
(f) MoS2
2.58. Each of the following compounds contains a metal that can exhibit more than one ionic charge. Name these compounds:
(a) NiCO3
(b) MoO3
(c) Co(NO3)2
(d) V2O5
(e) MnO2
(f) Fe2O3
2.59. The following ionic compounds are found in common household products. Write the formulas for each compound:
(a) potassium phosphate
(b) copper (II) sulfate
(c) calcium chloride
(d) titanium (IV) oxide
(e) ammonium nitrate
(f) sodium bisulfate (the common name for sodium hydrogen sulfate)
2.60. The following ionic compounds are found in common household products. Name each of the compounds:
(a) Ca(H2PO4)2
(b) FeSO4
(c) CaCO3
(d) MgO
(e) NaNO2
(f) KI
2.61. What are the IUPAC names of the following compounds?
(a) manganese dioxide
(b) mercurous chloride (Hg2Cl2)
(c) ferric nitrate [Fe(NO3)3]
(d) titanium tetrachloride
(e) cupric bromide (CuBr2)
Cumulative
2.62. Rare earth elements are extremely important to modern society[1]. They have also made recent news because the United States relies so heavily on imports from China[2]. In fact, even if the metals are mined in the United States, they are often shipped to China for processing; China controls 60% of the mined production, but 90% of the global processing.
(a) Where are the rare earth elements on the periodic table? (Hint: see Section 2.5.)
(b)
(c) It’s a long process between mining the rocks and obtaining the metal for use in electronics. The rock is removed, the ore is mined, a concentrate is recovered, and the metal is extracted. Nassar et al. (2023)[3] calculated a rock-to-metal ratio (RMR) to quantify how much rock it takes to extract the metal. Values range from 1.6 × 101 g rock/ 1 g metal to 3.6 × 103 g rock/ 1 g metal. Some examples are included in the table below:
| Element | Example Uses | Production (metric tons/yr) | RMR |
| lanthanum | creates alloys with other elements for camera lenses and lighting | 12,500 | 7.7 × 101 |
| cerium | catalytic converters | 24,000 | 2.3 × 101 |
| gadolinium | nuclear energy production as a neutron capture, MRIs, lasers, | 7,500 | 1.4 × 103 |
How much rock is needed to produce each of these metals every year? (Hint: 1 metric ton = 1,000 kg.) To put those numbers in perspective, let’s assume 1 African elephant is 5,900 kg, so how many elephants does each of these quantities equal?
(d) Turn your attention to waste: Not only do the tons and tons of leftover rock need to be put somewhere, but the extraction process also requires a lot of acids and toxic chemicals. This leads to environmental degradation and adverse health effects such as cancers, cardiovascular disease, and respiratory issues. Given our modern world, we need these elements; what might be a more sustainable way of extracting these metals?
- Ogasa, N. (2023, January 16). How rare earth elements' hidden properties make modern technology possible. Science News. ↵
- Funk, J. (2025, April 18). The US has a single rare earths mine. Chinese export limits are energizing a push for more. AP. ↵
- Nassar, N.T., et al. (2023). Rock-to-metal ratios of the rare earth elements. Journal of Cleaner Production, 405, 136958. ↵