Chapter 7 Chemical Bonding and Molecular Geometry
7.7 Molecular Polarity
Learning Objectives
By the end of this section, you will be able to:
- Assess the polarity of a molecule based on its bonding and structure
Molecular Polarity and Dipole Moment
As discussed previously, polar covalent bonds connect two atoms with differing electronegativities, leaving one atom with a partial positive charge (δ+) and the other atom with a partial negative charge (δ–), as the electrons are pulled toward the more electronegative atom. This separation of charge gives rise to a bond dipole moment. The magnitude of a bond dipole moment is represented by the Greek letter mu (µ) and is given by the formula shown here, where Q is the magnitude of the partial charges (determined by the electronegativity difference) and r is the distance between the charges:
This bond moment can be represented as a vector, a quantity having both direction and magnitude (Figure 7.26). Dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. The length of the arrow is proportional to the magnitude of the electronegativity difference between the two atoms.
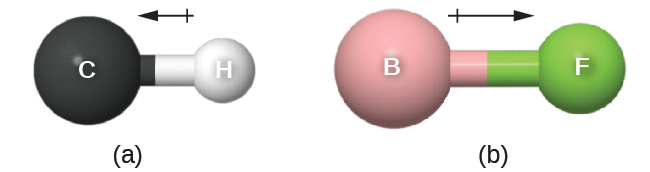
A whole molecule may also have a separation of charge, depending on its molecular structure and the polarity of each of its bonds. If such a charge separation exists, the molecule is said to be a polar molecule (or dipole); otherwise the molecule is said to be nonpolar. The dipole moment measures the extent of net charge separation in the molecule as a whole. We determine the dipole moment by adding the bond moments in three-dimensional space, taking into account the molecular structure.
For diatomic molecules, there is only one bond, so its bond dipole moment determines the molecular polarity. Homonuclear diatomic molecules such as Br2 and N2 have no difference in electronegativity, so their dipole moment is zero. For heteronuclear molecules such as CO, there is a small dipole moment. For HF, there is a larger dipole moment because there is a larger difference in electronegativity.
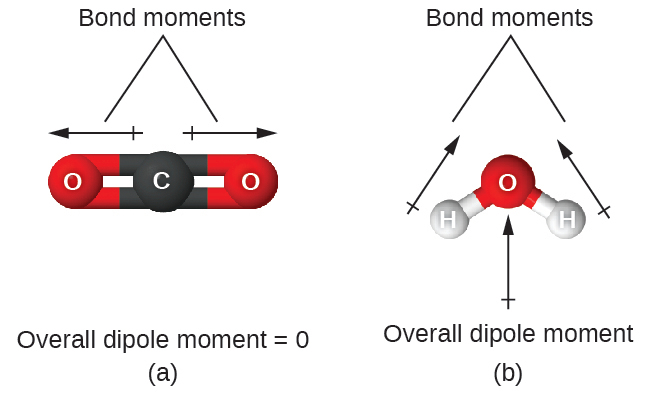
When a molecule contains more than one bond, the geometry must be taken into account. If the bonds in a molecule are arranged such that their bond moments cancel (vector sum equals zero), then the molecule is nonpolar. This is the situation in CO2 (Figure 7.27). Each of the bonds is polar, but the molecule as a whole is nonpolar. From the Lewis structure, and using VSEPR theory, we determine that the CO2 molecule is linear with polar C=O bonds on opposite sides of the carbon atom. The bond moments cancel because they are pointed in opposite directions. In the case of the water molecule (Figure 7.27), the Lewis structure again shows that there are two bonds to a central atom, and the electronegativity difference again shows that each of these bonds has a nonzero bond moment. In this case, however, the molecular structure is bent because of the lone pairs on O, and the two bond moments do not cancel. Therefore, water does have a net dipole moment and is a polar molecule (dipole).
The OCS molecule has a structure similar to CO2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is polar, we draw the molecular structure. VSEPR theory predicts a linear molecule: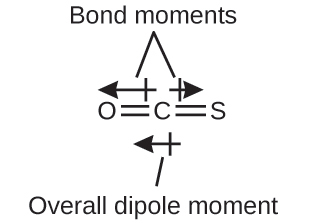
The C-O bond is considerably polar. Although C and S have very similar electronegativity values, S is slightly more electronegative than C, and so the C-S bond is just slightly polar. Because oxygen is more electronegative than sulfur, the oxygen end of the molecule is the negative end.
Chloromethane, CH3Cl, is a tetrahedral molecule with three slightly polar C-H bonds and a more polar C-Cl bond. The relative electronegativities of the bonded atoms is H < C < Cl, and so the bond moments all point toward the Cl end of the molecule and sum to yield a considerable dipole moment (the molecules are relatively polar).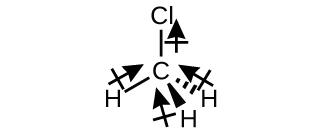
For molecules of high symmetry such as BF3 (trigonal planar), CH4 (tetrahedral), PF5 (trigonal bipyramidal), and SF6 (octahedral), all the bonds are of identical polarity (same bond moment) and they are oriented in geometries that yield nonpolar molecules (dipole moment is zero). Molecules of less geometric symmetry, however, may be polar even when all bond moments are identical. For these molecules, the directions of the equal bond moments are such that they sum to give a nonzero dipole moment and a polar molecule. Examples of such molecules include hydrogen sulfide, H2S (nonlinear), and ammonia, NH3 (trigonal pyramidal).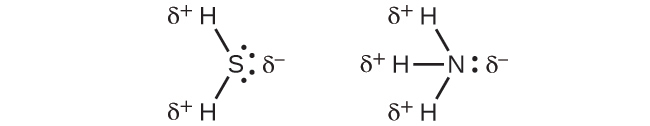
To summarize, to be polar, a molecule must:
- Contain at least one polar covalent bond.
- Have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel.
Example 7.18. Predicting Molecular Polarity
Click here for an explanation of this problem!
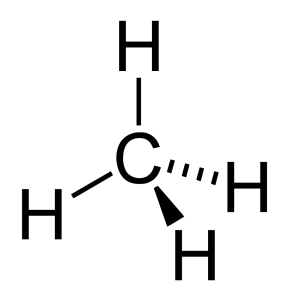
Methane is non-polar because it is highly symmetrical. Note that, additionally, C-H bonds are only weakly polar.
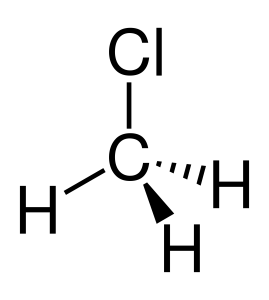
Chloromethane is polar because it has a polar C-Cl bond and is non-symmetrical. The dipole points to the Cl atom because it is the most electronegative.
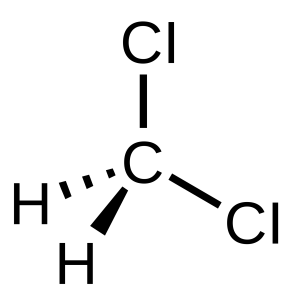
Dichloromethane (DCM) is polar because it has two polar C-Cl bonds and is non-symmetrical. The dipole points to the Cl atoms because they are the most electronegative.
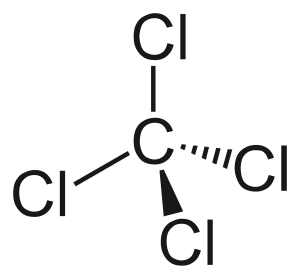
Tetrachloromethane (more commonly known as carbon tetrachloride) is non-polar, even though it contains four polar C-Cl bonds, because it is highly symmetrical.
Click here for an explanation of this problem!
![]()
Dioxygen is non-polar because its only bond is a non-polar bond.
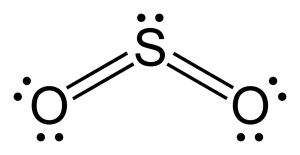
Silicon dioxide is polar because it contains two polar bonds (S=O) and is non-symmetrical. The dipole points towards the lone pair on silicon.
![]()
Carbon dioxide is non-polar, even though it contains two polar C=O bonds, because it is symmetrical.
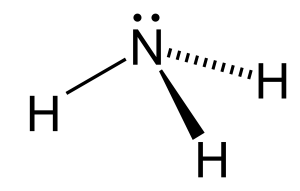
Ammonia is polar because it contains three polar bonds (N-H) and is non-symmetrical. The dipole points towards the lone pair on nitrogen.
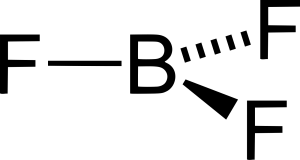
Boron trifluoride is non-polar, even though it contains three polar B-F bonds, because it is symmetrical. Remember: boron often disobeys the octet rule. B in BF3 has no lone pairs, so BF3 is trigonal planar and thus symmetrical.
Properties of Polar Molecules
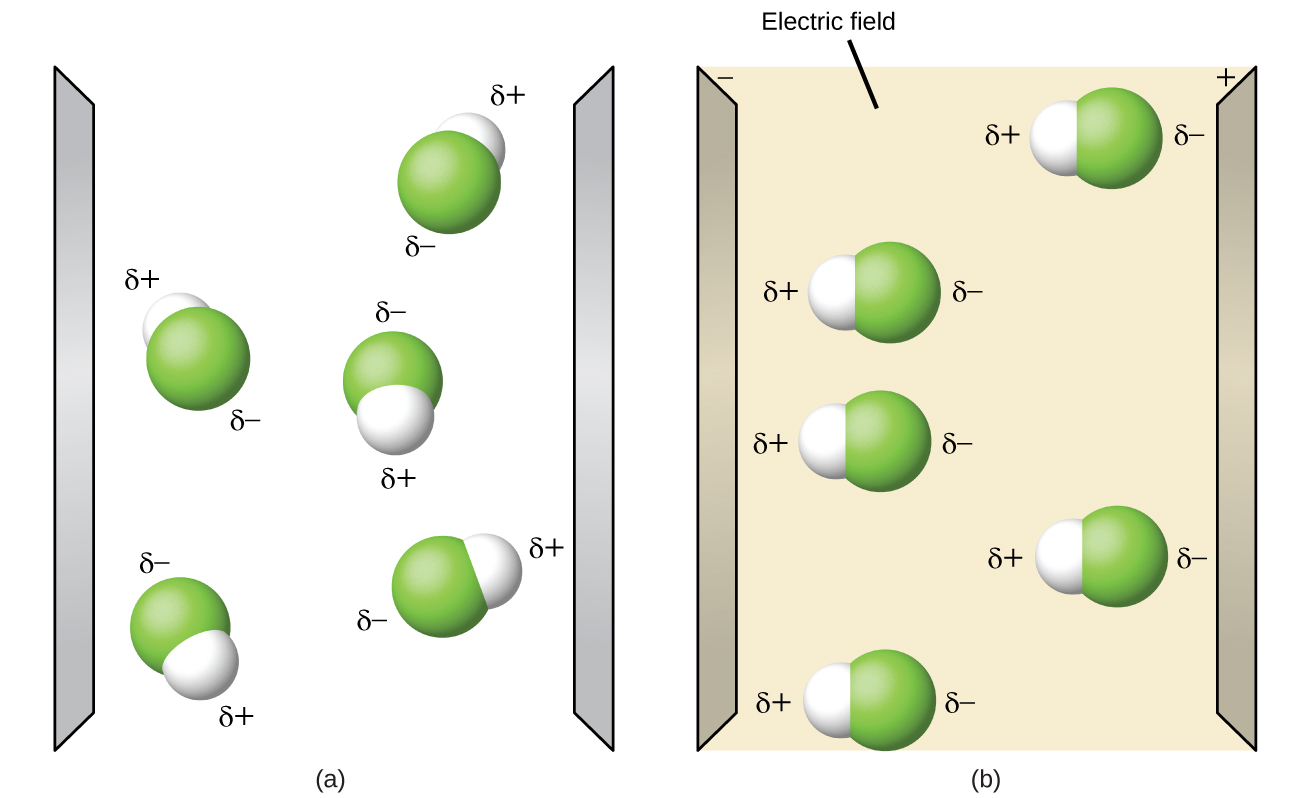
Polar molecules tend to align when placed in an electric field with the positive end of the molecule oriented toward the negative plate and the negative end toward the positive plate (Figure 7.28). We can use an electrically charged object to attract polar molecules, but nonpolar molecules are not attracted. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances.
Link to Learning
The molecule polarity simulation provides many ways to explore dipole moments of bonds and molecules.
Example 7.19. Polarity Simulations
Open the molecule polarity simulation and select the “Three Atoms” tab at the top. This should display a molecule ABC with three electronegativity adjustors. You can display or hide the bond moments, molecular dipoles, and partial charges on the right. Turning on the Electric Field will show whether the molecule moves when exposed to a field, similar to Figure 7.28.
Use the electronegativity controls to determine how the molecular dipole will look for the starting bent molecule if:
(a) A and C are very electronegative and B is in the middle of the range.
(b) A is very electronegative, and B and C are not.
Solution
(a) Molecular dipole moment points immediately between A and C.
(b) Molecular dipole moment points along the A–B bond, toward A.
Check Your Learning
Determine the partial charges that will give the largest possible bond dipoles.
Click here for the answer!
The largest bond moments will occur with the largest partial charges. The two solutions above represent how unevenly the electrons are shared in the bond. The bond moments will be maximized when the electronegativity difference is greatest. The controls for A and C should be set to one extreme, and B should be set to the opposite extreme. Although the magnitude of the bond moment will not change based on whether B is the most electronegative or the least, the direction of the bond moment will.
Assessing Polarity of Greenhouse Gases
Greenhouse gases are molecules in the atmosphere that absorb infrared (IR) radiation and re-emit energy, keeping heat in the Earth’s atmosphere. In order to absorb IR, the molecule must have a dipole at some point in its vibration, and thus they are polar molecules. Greenhouse gases participate in the Greenhouse Effect, which warms the Earth’s surface. Key greenhouse gases include carbon dioxide, methane, nitrous oxide, and water vapor, as well as synthetic gases like fluorinated gases. For more information about greenhouse gases, see Section 9.5.
Try drawing the Lewis structures of these greenhouse gases: nitrous oxide (N2O), water vapor (H2O), carbon dioxide (CO2), and methane (CH4). Determine whether you think each is a polar molecule.
Wait! You probably know that carbon dioxide is a greenhouse gas, but isn’t it nonpolar? Remember, gas molecules are constantly moving and that includes bending and stretching of the bonds. During vibration, molecules can undergo electron shifts, which can create dipoles. An induced dipole is all that is needed for the molecule to absorb radiation. A stagnant world does not account for vibrational changes. For instance, gases such as carbon dioxide and methane are nonpolar on paper but exhibit dipole changes during vibration that allow them to act as greenhouse gases. In contrast, symmetric diatomic molecules like N2 and O2 (which make up 99% of air) are unable to be greenhouse gases because their vibrations do not change the electron symmetry. For this chapter, we will focus on drawing polar and nonpolar molecules on a still world
