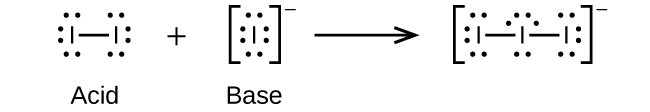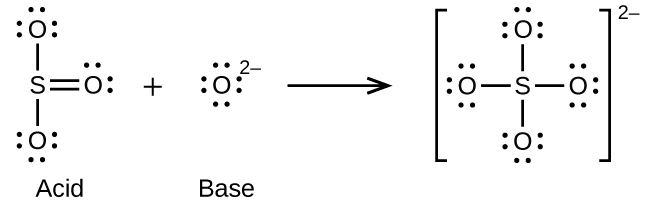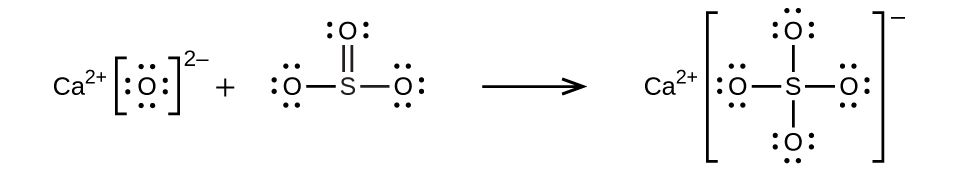Answer Keys to Selected Problems
Chapter 15 Key
15.3. There is no change. A solid has an activity of 1 whether there is a little or a lot.
15.5. The solubility of silver bromide at the new temperature must be known. Normally the solubility increases and some of the solid silver bromide will dissolve.
(a) LaF3(s) ⇌ La3+(aq) + 3 F−(aq) Ksp = [La3+] [F−]3
(b) CaCO3(s) ⇌ Ca2+(aq) + CO32−(aq) Ksp = [Ca2+] [CO32−]
(c) Ag2SO4(s) ⇌ 2 Ag+(aq) + SO42−(aq) Ksp = [Ag+]2 [SO42−]
(d) Pb(OH)3(s) ⇌ Pb2+(aq) + 2 OH−(aq) Ksp = [Pb2+] [OH−]2
15.11. (a) 1.77 × 10–7 (b) 1.6 × 10–6 (c) 2.2 × 10–9 (d) 7.91 × 10–22
15.13. (a) 2 × 10–2 M (b) 1.5 × 10–3 M (c) 2.27 × 10–9 M (d) 2.2 × 10–10 M
15.15. CaSO4∙2H2O is the most soluble Ca salt in mol/L, and it is also the most soluble Ca salt in g/L.
15.17. 4.8 × 10–3 M = [SO42−] = [Ca2+]; since this concentration is higher than 2.60 × 10–3 M, “gyp” water does not meet the standards.
(a) [Ag+] = [I–] = 1.3 × 10–5 M
(b) [Ag+] = 2.88 × 10–2 M, [SO42−] = 1.44 × 10–2 M
(c) [Mn2+] = 3.7 × 10–5 M, [OH–] = 7.4 × 10–5 M
(d) [Sr2+] = 4.3 × 10–2 M, [OH–] = 8.6 × 10–2 M
(e) [Mg2+] = 1.3 × 10–4 M, [OH–] = 2.6 × 10–4 M
15.23. (a) 1.45 × 10–4 (b) 8.2 × 10–55 (c) 1.35 × 10–4 (d) 1.18 × 10–5 (e) 1.08 × 10–10
15.35. (a) 2.25 L (b) 7.2 × 10–7 g
15.37. 100% of it is dissolved.
(a) [Ag+] = 6.4 × 10−9 M , [Cl−] = 0.025 M. Check: [latex]\frac {6.4 \times 10^{-9}}{0.025} \times 100 \% = 2.6 \times 10^{-5}\%[/latex], an insignificant change.
(b) [Ca2+] = 2.2 × 10−5 M , [F−] = 0.0013 M. Check: [latex]\frac {2.26 \times 10^{-5}}{0.00133} \times 100 \% = 1.70 \%[/latex]. This value is less than 5% and can be ignored.
(c) [SO42−] = 0.2238 M, [Ag+] = 7.4 × 10–3 M. Check: [latex]\frac {3.7 \times 10^{-3}}{0.2238} \times 100 \% = 1.64 \times 10^{-2} \%[/latex]. The condition is satisfied.
(d) [OH–] = 2.8 × 10–3 M, 5.7 × 10−12 M = [Zn2+]. Check: [latex]\frac {5.7 \times 10^{-12}}{2.8 \times 10^{-3}} \times 100 \% = 2.0 \times 10^{-7}\%[/latex], x is less than 5% of [OH–] and is therefore negligible.
(a) [Cl–] = 7.6 × 10−3 M. Check: [latex]\frac {7.6 \times 10^{-3}}{0.025} \times 100 \% = 30 \%[/latex]. This value is too large to drop x. Therefore solve by using the quadratic equation: [Ti+] = 3.1 × 10–2 M, [Cl–] = 6.1 × 10–3
(b) [Ba2+] = 7.7 × 10–4 M. Check: [latex]\frac {7.7 \times 10^{-4}}{0.0313} \times 100 \% = 2.4\%[/latex]. Therefore, the condition is satisfied. [Ba2+] = 7.7 × 10–4 M, [F–] = 0.0321 M
(c) Mg(NO3)2 = 0.02444 M, [C2O42−] = 2.9 × 10−5 M. Check: [latex]\frac {2.9 \times 10^{-5}}{0.02444} \times 100 \% = 0.12 \%[/latex]. The condition is satisfied; the above value is less than 5%. [C2O42−] = 2.9 × 10−5 M, [Mg2+] = 0.0244 M
(d) [OH–] = 0.0501 M, [Ca2+] = 3.15 × 10–3. Check: [latex]\frac {3.15 \times 10^{-3}}{0.050} \times 100 \% = 6.28 \%[/latex]. This value is greater than 5%, so a more exact method, such as successive approximations, must be used. [Ca2+] = 2.8 × 10–3 M, [OH–] = 0.053 × 10–2 M
15.43. The changes in concentration are greater than 5% and thus exceed the maximum value for disregarding the change.
15.45. (a) Hg22+ and Cu2+: add SO42− (b) SO42− and Cl–: add Ba2+ (c) Hg2+ and Co2+: add S2– (d) Zn2+ and Sr2+: add OH– until [OH–] = 0.050 M (e) Ba2+ and Mg2+: add SO42− (f) CO32− and OH–: add Ba2+
15.53. (a) 3.1 × 10–11 (b) [Cu2+] = 2.6 × 10–3, [IO3−] = 5.3 × 10–3
15.57. Mg(OH)2(s) ⇌ Mg2+ + 2 OH− Ksp = [Mg2+] [OH−]2
1.23 × 10−3 g Mg(OH)2
15.59. MnCO3 will form first since it has the smallest Ksp value among these homologous compounds and is therefore the least soluble. MgCO3∙3H2O will be the last to precipitate since it has the largest Ksp value and is the most soluble. Ksp value.
15.62. When the amount of solid is so small that a saturated solution is not produced.
| Cd(CN)42– | ⇌ | Cd2+ | + | 4 CN– | |
| I (M) | 0.250 | 0 | 0 | ||
| C (M) | –x | +x | +4x | ||
| E (M) |
0.250 – x | x | 4x |
[Cd2+] = 9.5 × 10–5 M, [CN–] = 3.8 × 10–4 M
15.70. [Co3+] = 3.0 × 10–6 M, [NH3] = 1.8 × 10–5 M
(a)
(b)
(c)
(d)
(e)
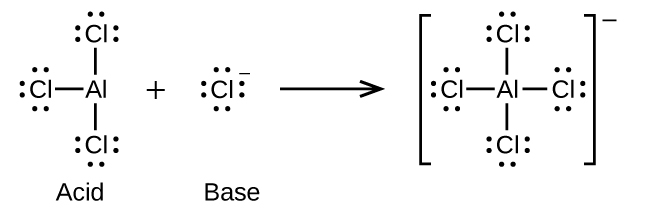
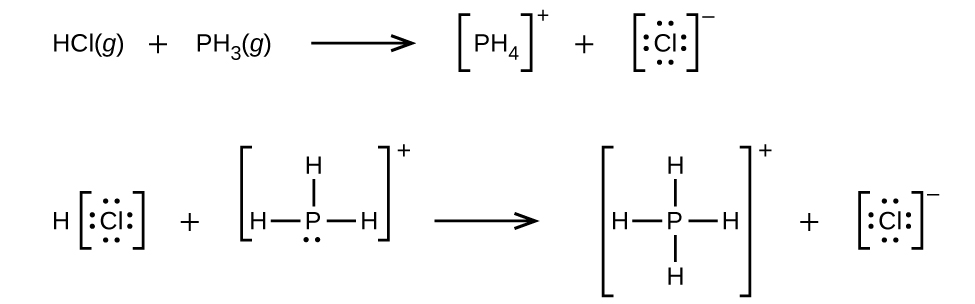
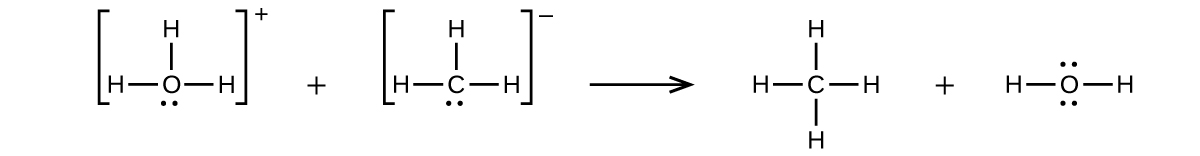
(a)
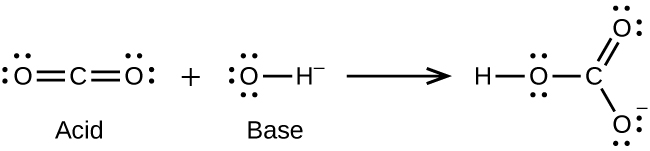
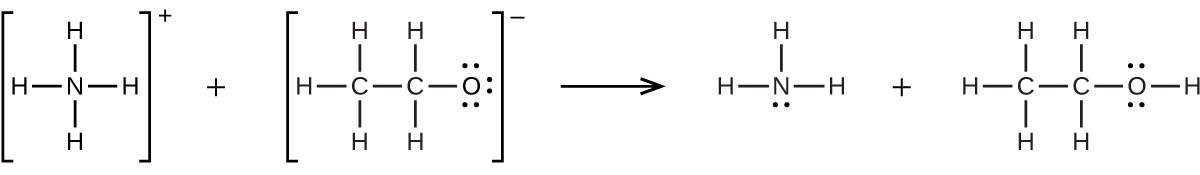
(b) H3O+ + CH3− → CH4 + H2O
(c) CaO + SO3 → CaSO4
(d) NH4+ + C2H5O− → C2H5OH + NH3
15.82. HNO3(l) + HF(l) → H2NO3+ + F− HF(l) + BF3(g) → H+ + BF4−
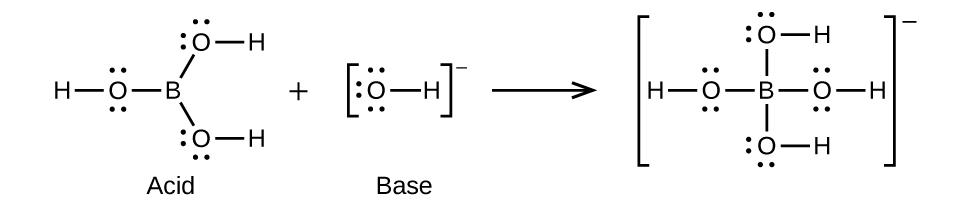
15.84. (a) H3BO3 + H2O → H4BO4− + H+ (b) The electronic and molecular shapes are the same—both tetrahedral. (c) The tetrahedral structure is consistent with sp3 hybridization.
15.93. [OH−] = 4.5 × 10−6 [Al3+] = 2 × 10–16 (molar solubility)
15.95. [SO42−]=0.049 M [Ba2+] = 4.7 × 10–7 (molar solubility)
15.97. [OH–] = 7.6 × 10−3 M [Pb2+] = 2.1 × 10–11 (molar solubility)
(a) Ksp = [Mg2+][F–]2 = (1.21 × 10–3)(2 × 1.21 × 10–3)2 = 7.09 × 10–9
(b) 7.09 × 10–7 M
(c) Determine the concentration of Mg2+ and F– that will be present in the final volume using M1V1 = M2V2.
M2 = 1.33 × 10–3 M
Compare the value of the ion product Qsp with Ksp. If this value is larger than Ksp, precipitation will occur.
(d) MgF2 is less soluble at 27°C than at 18°C. Because added heat acts like an added reagent, when it appears on the product side, the Le Chatelier’s principle states that the equilibrium will shift to the reactants’ side to counter the stress. Consequently, less reagent will dissolve. This situation is found in our case. Therefore, the reaction is exothermic.
15.103. BaF2, Ca3(PO4)2, ZnS; each is a salt of a weak acid, and the [H3O+] from perchloric acid reduces the equilibrium concentration of the anion, thereby increasing the concentration of the cations.
(a) 9.9 × 10–11 M
(b) 1.1 × 10–9 g Fe(OH)3 per 100 g solvent
(c) 2.6 × 10–36 M. The presence of the common ion has depressed the solubility (increased amount of product, shifts equilibrium towards more solid, thus decrease solubility)—this time by almost 25 orders of magnitude. You can get this [OH–] from any base. Placing this salt in a basic solution will create this common ion effect.
(d) In an acidic solution, the solubility of Fe(OH)₃ increases because H⁺ ions neutralize OH–, reducing its concentration and shifting the equilibrium toward dissolving more solid Fe(OH)₃.
(a) Solubility is 6.36 × 10–5 M (5°C), 5.75 × 10–5 M (25°C), 5.13 × 10–5 M (40°C), and 2.75 × 10–5 M (90°C).
(b) The solubility decreases by about 11% when the temperature increases from 25°C to 40°C. As we can see, solubility decreases when temperature increases.
(c) As global temperatures rise, ocean temperatures increase, which causes CaCO₃ to become more soluble in seawater. This weakens the strength of the coral reef system because corals have a harder time building and maintaining their skeletons, and makes them more vulnerable to erosion, bleaching, and storm damage. Coral reefs are an essential aspect of marine life biodiversity; therefore, altering the reefs alters the entire ecosystem. (Note: This doesn't take into account the added acid coming from carbon dioxide—forming carbonic acid when dissolved in water—that will react away carbonate bases.)