Answer Keys to Selected Problems
Chapter 19 Key
19.1. (a) Sc: [Ar]4s23d 1 (b) Ti: [Ar]4s23d 2 (c) Cr: [Ar]4s13d 5 (d) Fe: [Ar]4s23d 6 (e) Ru: [Kr]5s24d 6
19.3. (a) La: [Xe]6s25d 1, La3+: [Xe] (b) Sm: [Xe]6s24f 6, Sm3+: [Xe]4f 5 (c) Lu: [Xe]6s24f 145d 1, Lu3+: [Xe]4f 14
19.5. Al is used because it is the strongest reducing agent and the only option listed that can provide sufficient driving force to convert La(III) into La.
19.9. The CaSiO3 slag is less dense than the molten iron, so it can easily be separated. Also, the floating slag layer creates a barrier that prevents the molten iron from exposure to O2, which would oxidize the Fe back to Fe2O3.
19.15. E° = −0.6 V; E° is negative, so this reduction is not spontaneous. E° = +1.1 V
(a) Fe(s) + 2 H3O+(aq) + SO42−(aq) → Fe2+(aq) + SO42−(aq) + H2(g) + 2 H2O(l)
(b). FeCl3(aq) + 3 Na+(aq) + 3 OH−(aq) →Fe(OH)3(s) + 3 Na+(aq) + 3 Cl−(aq)
(c). Mn(OH)2(s) + 2 H3O+(aq) + 2 Br−(aq) → Mn2+(aq) + 2 Br−(aq) + 4 H2O(l)
(d). 4 Cr(s) + 3 O2(g) → 2 Cr2O3(s)
(e). Mn2O3(s) + 6 H3O+(aq) + 6 Cl−(aq) → 2 MnCl3(s) + 9 H2O(l)
(f) Ti(s) + xs F2(g) → TiF4(g)
(a) Cr2(SO4)3(aq) + 2 Zn(s) + 2 H3O+(aq) → 2 Zn2+(aq) + H2(g) + 2 H2O(l) + 2 Cr2+(aq) + 3 SO42−(aq)
(b) 4 TiCl3(s) + CrO42−(aq) + 8 H+(aq) → 4 Ti4+(aq) + Cr(s) + 4 H2O(l) + 12 Cl−(aq)
(c) In acid solution between pH 2 and pH 6, CrO42− forms HCrO4−, which is in equilibrium with dichromate ion. The reaction is 2 HCrO4−(aq) → Cr2O72−(aq) + H2O(l).
At other acidic pHs, the reaction is 3 Cr2+(aq) + CrO42−(aq) + 8 H3O+(aq) → 4 Cr3+(aq) + 12 H2O(l).
(d) 8 CrO3(s) + 9 Mn(s) [latex]\xrightarrow {\Delta}[/latex] 4 Cr2O3(s) + 3 Mn3O4(s)
(e) CrO(s) + 2 H3O+(aq) + 2 NO3−(aq) → Cr2+(aq) + 2 NO3−(aq) + 3 H2O(l)
(f) CrCl3(s) + 3 NaOH(aq) → Cr(OH)3(s) + 3 Na+(aq) + 3 Cl−(aq)
(a) 3 Fe(s) + 4 H2O(g) → Fe3O4(s) + 4 H2(g)
(b) 3 NaOH(aq) + Fe(NO3)3(aq) [latex]\xrightarrow {H_2O}[/latex] Fe(OH)3(s) + 3 Na+(aq) + 3 NO3−(aq)
(c) MnO4− + 5 Fe2+ + 8 H+ → Mn2+ + 5 Fe3 + 4 H2O
(d) Fe(s) + 2 H3O+(aq) + SO42−(aq) → Fe2+(aq) + SO42−(aq) + H2(g) + 2 H2O(l)
(e) 4 Fe2+(aq) + O2(g) + 4 HNO3(aq) → 4 Fe3+(aq) + 2 H2O(l) + 4 NO3−(aq)
(f) FeCO3(s) + 2 HClO4(aq) → Fe(ClO4)2(aq) + H2O(l) + CO2(g)
(g) 3 Fe(s) + 2 O2(g) [latex]\xrightarrow {\Delta}[/latex] Fe3O4(s)
19.23. As CN− is added, Ag+(aq) + CN−(aq) → AgCN(s).
As more CN− is added:
19.25. (a) Sc3+ (b) Ti4+ (c) V5+ (d) Cr6+ (e) Mn4+ (f) Fe2+ and Fe3+ (g) Co2+ and Co3+ (h) Ni2+ (i) Cu+
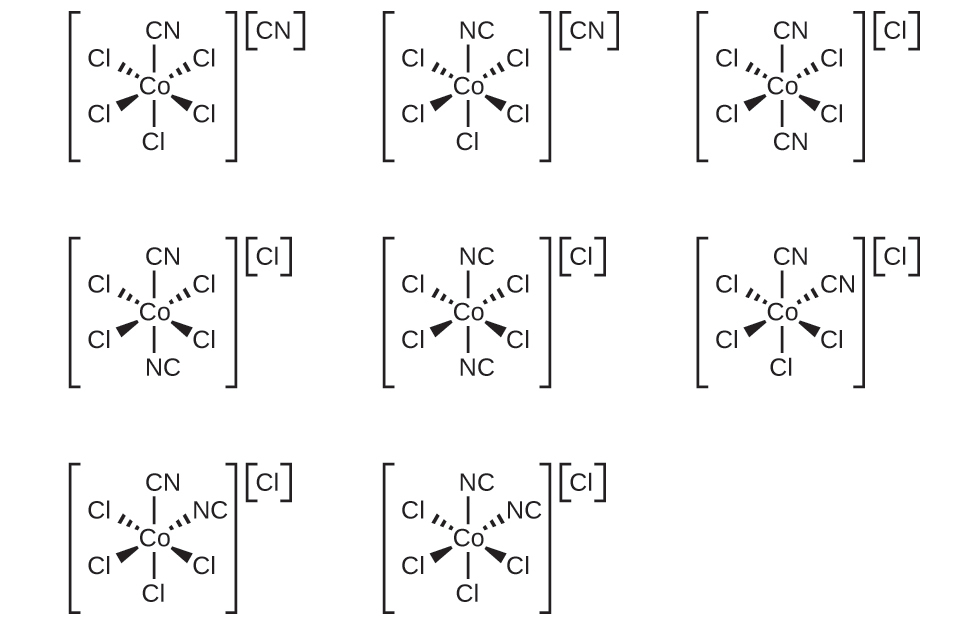
19.27. (a) 4, [Zn(OH)4]2− (b) 6, [Pd(CN)6]2− (c) 2, [AuCl2]− (d) 4, [Pt(NH3)2Cl2] (e) 6, K[Cr(NH3)2Cl4] (f) 6, [Co(NH3)6][Cr(CN)6] (g) 6, [Co(en)2Br2]NO3
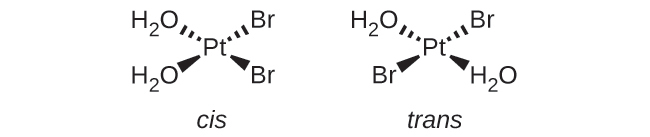
(a) [Pt(H2O)2Br2]:
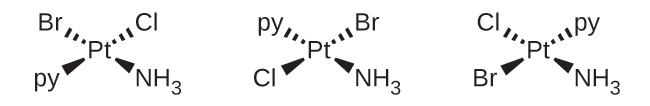
(b) [Pt(NH3)(py)(Cl)(Br)]:
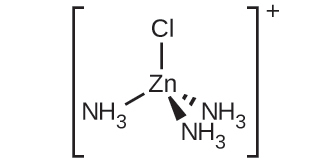
(c) [Zn(NH3)3Cl]+ :
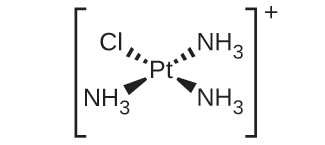
(d) [Pt(NH3)3Cl]+ :
(e) [Ni(H2O)4Cl2]:
(f) [Co(C2O4)2Cl2]3−:
19.31. (a) tricarbonatocobaltate (III) ion (b) tetraaminecopper(II) ion (c) tetraaminedibromocobalt (III) sulfate (d) tetraamineplatinum (II) tetrachloroplatinate (II) (e) tris-(ethylenediamine)chromium (III) nitrate (f) diaminedibromopalladium(II) (g) potassium pentachlorocuprate (II) (h) diaminedichlorozinc (II)
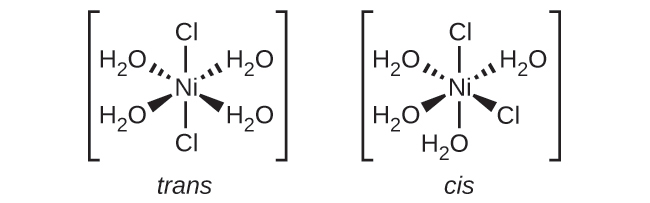
19.33. (a) none (b) none (c) The two Cl ligands can be cis or trans. When they are cis, there will also be an optical isomer.
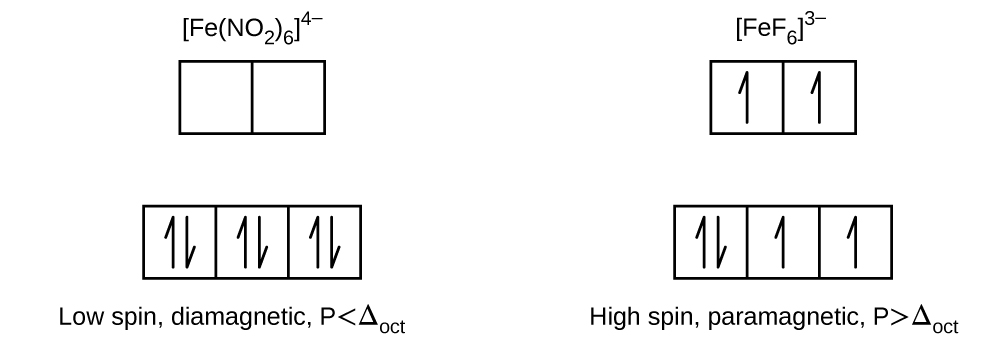
19.39. [Co(H2O)6]Cl2 with three unpaired electrons
19.41. (a) 4 (b) 2 (c) 1 (d) 5 (e) 0
19.43. (a) [Fe(CN)6]4− (b) [Co(NH3)6]3+ (c) [Mn(CN)6]4−
19.45. The complex does not have any unpaired electrons. The complex does not have any geometric isomers, but the mirror image is nonsuperimposable, so it has an optical isomer.