Welcome
Welcome to General Chemistry 3e: OER for Inclusive Learning, a Pressbooks resource built from the Chemistry 2e OpenStax resource. As an open educational resource (OER), this textbook allows students to access high-quality learning materials at little to no cost while maintaining the highest standards of academic rigor. Building on Chemistry 2e, Chemistry 3e increases student accessibility, inclusivity, and engagement to enhance each student's sense of belonging. Social justice content is included to increase students’ awareness of the role chemistry can play in addressing social and environmental inequities.
Acknowledgment of FIPSE Funding
The modifications from Chemistry 2e were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education. The content does not necessarily represent the policy of the U.S. Department of Education, and you should not assume endorsement by the federal government. For more information about the OER for Social Justice grant, visit our project [Website].
About Chemistry 3e
Chemistry 3e is designed to meet the content requirements of the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them.
Coverage and Scope
Our Chemistry 3e textbook adheres to the scope and sequence of most general chemistry courses nationwide. We strive to make chemistry, as a discipline, interesting and accessible to students. With this objective in mind, the content of this textbook has been developed and arranged to provide a logical progression from fundamental to more advanced concepts of chemical science. Topics are introduced within the context of familiar experiences whenever possible, treated with an appropriate rigor to satisfy the intellect of the learner, and reinforced in subsequent discussions of related content. The organization and pedagogical features were developed and vetted with feedback from chemistry educators dedicated to the project.
Customization
Chemistry 3e is licensed under a Creative Commons Attribution 4.0 International (CC-BY-NC-SA) license, which means that you can distribute, remix, and build upon the content, as long as you provide attribution to Pressbooks and its content contributors and you do not use the material commercially. Please also use the same attribution for your new document.
Because our book is openly licensed, you are free to use the entire book or pick and choose the sections that are most relevant to the needs of your course. Feel free to remix the content by assigning your students certain chapters and sections in your syllabus, in the order that you prefer. You can even provide a direct link in your syllabus to any sections you are using.
Errata
This textbook underwent a rigorous review process. However, like any professional-grade textbook, errors sometimes occur. Since this book is web based, we can make updates periodically when deemed pedagogically necessary. If you have a correction to suggest, submit it through this link [Website]. Subject matter experts review all errata suggestions.
Changes to the Second Edition
This book builds on Chemistry 2e to include innovative interactive exercises and real-world applications designed to promote inclusive students learning and enhance daily relevance. Chemistry 3e has been revised to incorporate more clear, current, and dynamic explanations. Substantial improvements have been made in the figures, illustrations, and example exercises to support the text narrative.
Interactives That Engage
We have embedded numerous H5P interactives. Below are a few examples.
Manipulating 3-D structures:
Identifying types of ions:
Ranking the atomic radius:
Marking the identity of ions:
Drag-and-drop identification:
Multistep questions with hints:
Walk-through of procedural steps in problem solving:
Check Your Learning
Using the ICE table you developed in the quiz above, determine the equilibrium concentrations for CH3CO2H, C2H5OH, CH3CO2C2H5, and H2O.
Click here to see the answers and a walkthrough!
First, we calculate Q to determine which direction the reaction will proceed:
[latex]Q\ =\ \frac{\left[CH_3CO_2C_2H_5\right]\left[H_2O\right]}{\left[CH_3CO_2H\right]\left[C_2H_5OH\right]}=\frac{\left(0.40\right)\left(0.40\right)}{\left(0.15\right)\left(0.15\right)}=7.1[/latex]
Since Q > K, the reaction will proceed to the left (towards the reactants). Develop an ICE table that reflects this:
| C2H5OH | + | CH3CO2H | ⇌ | CH3CO2C2H5 | + | H2O | |
| I (M) | 0.15 | 0.15 | 0.40 | 0.40 | |||
| C (M) | +x | +x | −x | −x | |||
| E (M) | 0.15 + x | 0.15 + x | 0.40 − x | 0.40 − x |
Substituting in our equilibrium expressions into Kc, we can solve for x:
[latex]K_c\ =\ \frac{\left[CH_3CO_2C_2H_5\right]\left[H_2O\right]}{\left[CH_3CO_2H\right]\left[C_2H_5OH\right]}[/latex]
[latex]4.0\ =\ \frac{\left(0.40-x\right)\left(0.40\ -\ x\right)}{\left(0.15\ +\ x\right)\left(0.15\ +x\right)}[/latex]
[latex]4.0\ =\ \frac{0.16\ -0.80x\ +x^2}{0.0225\ +0.30x+x^2}[/latex]
[latex]0.09\ +\ 1.2x\ +4.0x^2\ =0.16\ -0.80x+x^2[/latex]
[latex]3.0x^2+2.0x-0.07=0[/latex]
Using the quadratic formula, we find:
[latex]x\ =0.033[/latex]
and
[latex]x\ =-0.70[/latex]
We use x = 0.033 because x = -0.70 would yield negative concentrations, which are non-physical. Filling out our ice chart with numerical values, we get:
| C2H5OH | + | CH3CO2H | ⇌ | CH3CO2C2H5 | + | H2O | |
| I (M) | 0.15 | 0.15 | 0.40 | 0.40 | |||
| C (M) | +0.03 | +0.03 | −0.03 | −0.03 | |||
| E (M) | 0.18 | 0.18 | 0.37 | 0.37 |
We can check our answer by substituting the equilibrium concentrations into the Kc expression:
[latex]K_c=\frac{\left(0.37\right)\left(0.37\right)}{\left(0.18\right)\left(0.18\right)}=4.2[/latex]
Check Your Learning
A 1.00-L flask is filled with 1.00 mole of H2 and 2.00 moles of I2. The value of the equilibrium constant for the reaction of hydrogen and iodine reacting to form hydrogen iodide is 50.5 under the given conditions.
What are the equilibrium concentrations of H2, I2, and HI in moles/L?
Click here to see the answers and a walkthrough!
Since there are initially no mols of the product (HI), Q = 0 and the reaction must proceed to the right. We create an ICE table that reflects this:
| H2(g) | + | I2(g) | ⇌ | 2 HI(g) | |
| I (M) | 1.00 | 2.00 | 0 | ||
| C (M) | −x | -x | +2x | ||
| E (M) | 1.00 −x | 2.00 - x | 2x |
Substitution of the equilibrium expressions into the Kc expression allows us to solve for x:
[latex]K_c=\frac{\left[HI\right]^2}{\left[H_2\right]\left[I_2\right]}[/latex]
[latex]50.5\ =\frac{\left(2x\right)^2}{\left(1.00-x\right)\left(2.00-x\right)}=\frac{4x^2}{2.00-3.00x+x^2}[/latex]
[latex]101\ -\ 152x\ +\ 50.5x^2=4x^2[/latex]
[latex]46.5x^2-152x+101=0[/latex]
Using the quadratic formula, we find:
x = 2.3
and
x = 0.94
We choose x = 0.94 because x = 2.3 yields negative concentrations. Filling out our ice chart with numerical values, we get:
| H2(g) | + | I2(g) | ⇌ | 2 HI(g) | |
| I (M) | 1.00 | 2.00 | 0 | ||
| C (M) | −0.94 | -0.94 | +2(0.94) | ||
| E (M) | 0.06 | 1.06 | 1.88 |
We can check our answer by substituting the equilibrium concentrations into the Kc expression:
[latex]K_c=\frac{\left(1.88\right)^2}{\left(0.06\right)\left(1.06\right)}=55.5[/latex]
Although not exact, this value is close to the actual value of Kc (50.5).
In addition to making reading the textbook more active and engaging, the H5P questions throughout the textbook can be linked with certain learning management systems (LMSs) to provide credit for completion or accurate responses. Using H5Ps as LMS-linked assessments can incentivize students to complete the assigned reading.
Real-World Connections
To engage students, we want to provide real-world examples and connect the content to everyday life. Some examples include:
Assessing Polarity of Greenhouse Gases
Greenhouse gases are molecules in the atmosphere that absorb infrared radiation (IR) and re-emit energy, keeping heat in the Earth's atmosphere. In order to absorb IR, the molecule must have a dipole at some point in its vibration, thus they are polar molecules. Greenhouse gases participate in the greenhouse effect, which warms the Earth’s surface. Key greenhouse gases include carbon dioxide, methane, nitrous oxide, and water vapor, as well as synthetic gases like fluorinated gases. For more information about greenhouse gases, see Section 9.5.
Try drawing the Lewis structures of these greenhouse gases: nitrous oxide (N2O), water vapor (H2O), carbon dioxide (CO2), and methane (CH4). Determine whether you think each is a polar molecule.
Wait! You probably know that carbon dioxide is a greenhouse gas, but isn't it nonpolar? Remember, gas molecules are constantly moving, and that includes bending and stretching of the bonds. During vibration, molecules can undergo electron shifts, which can create dipoles. An induced dipole is all that is needed for the molecule to absorb radiation. A stagnant world does not account for vibrational changes. For instance, gases such as carbon dioxide and methane are nonpolar on paper but exhibit dipole changes during vibration that allow them to act as greenhouse gases. In contrast, symmetric diatomic molecules like N2 and O2 (which make up 99% of air) are unable to be greenhouse gases because their vibrations do not change the electron symmetry. For this chapter, we will focus on drawing polar and nonpolar molecules on a still world.
Connecting Questions to Different Cultures
11.77. The authors are writing this in Los Angeles, a land that was originally cared for by the Tongva peoples. We are grateful to have the opportunity to live, study, create, and be in Tovaangar—an area that encompasses the Los Angeles Basin and southern Channel Islands. Let's explore some uses of the land by the Tongva people.
(a) A te'aat is a boat made by tying pine driftwood together using vegetable fiber cords and glued with asphalt that washed up from underwater seeps or that pooled in what is now known as the La Brea Tar Pits. The boat was then stained with red ochre and sealed with pine pitch.
(i) Asphalt is made up of hydrocarbons from ancient organic matter (like plankton). Given what you know about solubility, why does this protect the boat from leaks?
(ii) Ochre has been used as a dye since the dawn of human history; it dates back almost 300,000 years in Africa. Ochre is iron(III) oxide mixed with clay and sand and is derived from iron-rich soils. What is the chemical formula for ochre?
(b) Acorns from oak trees were a staple food source for Native Americans living in Southern California. Unfortunately, raw acorns are very bitter due to the tannic acid (C76H52O46). Thus, the Tongva people shelled the acorns, ground them up, and washed the meal with hot water. Given what you know about solubility, why would water work to wash out the tannin? (Hint: Think about the physical states that we always use for acids.) Why would hot water work better than cold water?
A "Portrait of a Chemist" in Each Chapter
Portrait of a Chemist
Omowunmi Sadik
Dr. Omowunmi "Wunmi" Sadik is a Nigerian-American analytical and environmental chemist whose work combines cutting-edge research with a passion for global sustainability. She earned her BS in chemistry from the University of Lagos (Nigeria) and her PhD in chemistry from the University of Wollongong (Australia), and she went on to become a professor at SUNY Binghamton. She is also the co-founder of the Sustainable Nanotechnology Organization.

Dr. Sadik is best known for her development of biosensors—analytical devices that use biological components to detect specific chemicals like drugs, explosives, and environmental toxins—in the environment or in biological systems.
"How Sciences Interconnect" in Each Chapter
How Sciences Interconnect
Greenhouse Gases and Climate Change
The thin skin of our atmosphere keeps Earth from being an ice planet and makes it habitable. In fact, this is due to less than 0.5% of the gas molecules present in our atmosphere. Of the energy from the sun that reaches the Earth, about 30% is reflected back into space, with the rest absorbed by the atmosphere and the surface of the Earth. Some of the energy that the Earth absorbs is re-emitted as infrared (IR) radiation, a portion of which passes through the atmosphere back into space. Most of this IR radiation, however, is absorbed by certain atmospheric gases, which in turn re-emit the radiation back towards Earth's surface (Figure 9.25). This phenomenon, known as the greenhouse effect, maintains global temperatures within the range needed to sustain life on Earth. Without our atmosphere, the Earth's average temperature would be lower by more than 30°C (nearly 60°F). The major greenhouse gases (GHGs) are water vapor, carbon dioxide, methane, nitrous oxide, and ozone..
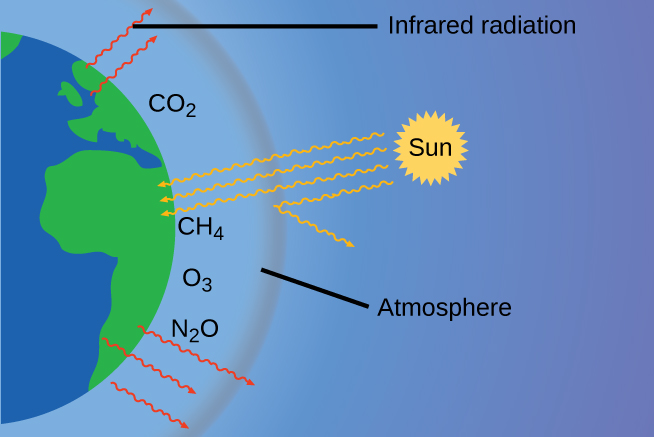
While historical records credit Irish physicist John Tyndall for being the first to show that CO2 and H2O can absorb IR radiation in 1859, this acclaim should go to American amateur scientist Eunice Newton Foote and her work in 1856 (Figure 9.26). Foote submitted her findings to the American Association for the Advancement of Science (AAAS) and Joseph Henry presented her research on her behalf at the meeting. No woman had ever presented before; it is not obvious whether a woman scientist would have been given the opportunity. Her work was written up in Scientific American, the Annual of Scientific Discovery, and a German publication where they mistook her for a man. Foote even speculated about how this warming could affect our atmosphere: "An atmosphere of that gas would give to our earth a much higher temperature; and if there once was, as some suppose, a larger proportion of that gas in the air, an increased temperature must have accompanied it...[1]" It's unclear if Tyndall was aware of Foote's accomplishment. Because he had access to the latest scientific equipment of the day, he was able to advance these discoveries much further than Foote had..

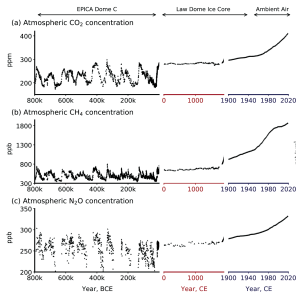
Climate scientists call the factors (both natural and anthropogenic) that influence Earth's energy balance radiative forcings. Greenhouse gases represent the most dominant anthropogenic forcing. In fact, the modern impact of all GHGs on Earth's increase in temperature is 2.5 times larger than all other forcings combined. This modern increase in GHG emissions started in the mid-1800s and is attributed to human activity such as agriculture, mining, industry, and transportation. Data collected from Antarctic ice cores shows that the concentrations of GHGs, such as carbon dioxide, methane, and nitrous oxide, are significantly higher than at any point in the past 1 million years (Figure 9.27). In recent years, the GHG concentrations are accelerating. For example, CO2 concentration has increased from preindustrial levels of ~280 ppm to more than 420 ppm today..
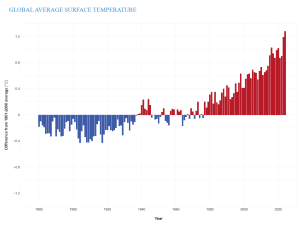
With the increase in GHG concentrations, the Earth's energy balance has changed, thus altering the Earth's climate. In fact, Earth's temperature has risen by an average of 0.06°C (0.11°F) per decade since 1850, or about 1°C (2°F) in total. The 10 warmest years in the historical record have all occurred in the past decade (2014 to 2023) (Figure 9.28). It is important to realize that an average increase in global temperature does not mean that every place is 1°C warmer today than it was in 1850. In fact, many regions have experienced a little warming and others have cooled. Yet there are places, particularly in the higher latitudes, that have experienced greater-than-average warming.
End-of-Chapter Cumulative Problems
Some examples include:
1.106. An average 1-kW solar panel is 68.42 ft2 and can produce 1,642 kWh. The surface area of California is 163,696 mi2 and its population is 39.43 million people. If each person uses 6.32 × 103 kWh/yr, what percent of the total area of California will be needed to cover with solar panels to suit the electricity needs of everyone in California for one year? (Hint: 5,280 ft = 1 mile.)
5.87. While we still rely on fossil fuels for the majority of our energy, burning natural gas (which is mainly methane) emits less CO2 than burning coal or oil.
(a) How much energy does the complete combustion of 1 mole of methane produce? Assume the water produced is steam. Refer to thermochemistry data found in Appendix G.
(b) Let's assume all of the energy produced by methane combustion goes to warming the water in a swimming pool. (In reality, much of the energy is lost through the transmission of electricity from the power plant.) How much energy would it take to heat a 2.00 × 104 (a.k.a. 20,000)-gallon backyard swimming pool from 52°F to 78°F? Assume the density of water is exactly 1 g/mL. Temperature conversions can be found in Section 1.6.
(c) If the cost of natural gas averaged $14.44 per 1,000 cubic ft in May 2024[2], how much would it cost to heat this swimming pool? The density of methane is 18.6 g/ft3.
11.76. Our oceans are made of seawater, which is a saltwater solution. The total volume of the entire ocean is 1.3375 million cubic kilometers. The average temperature of the entire ocean is 3.5°C (because most of the ocean is below the surface and not directly warmed by the sun), but the oceans are slowly warming due to global climate change. When people think about sea level increasing, they mostly think about the melting of glacial ice, but half of the projected sea level increase will simply come from thermal expansion—the fact that warm water takes up more volume. Below is a table of seawater densities:
|
Temperature (°C) |
Density (kg/m3) |
| 3.5 | 1,027.8832 |
| 10 | 1,027.0000 |
(a) Calculate the mass of seawater in the entire ocean.
(b) Assuming the mass of the ocean stays the same (calculated in (a)), what is the volume of the ocean when temperatures increase to 10°C?
(c) What is the change in volume between 3.5°C and 10°C?
(d) The Amazon River is the largest river in the world based on the vast quantity of water that it discharges into the ocean. If the Amazon River releases 2.09 × 105 m3/s into the Atlantic Ocean, how many days will it take the Amazon to discharge the volume change calculated in part (c)?
(e) How would these values (volume (c) and time (d)) differ if the ocean was made of fresh water? Refer to Appendix E for properties of pure water.
(f) How does salt water differ in terms of freezing point, boiling point, and density compared to fresh water? Why?
(g) Do a little research to figure out who might be affected by rising sea levels.
14.96. As seen in the introduction, acid rain is a result of air pollution from non-renewable energy sources. Acid rain primarily contains sulfuric acid (H2SO4) and nitric acid (HNO3) due to the dissolution of SO2 and NOx gases in atmospheric moisture. If 9.2 × 10−8 atm of SO2 were released into the atmosphere by a coal plant and reacted with water vapor in the atmosphere to form acidic rain, what is the pH of the rain?
First we have to consider the equilibrium between the gas and aqueous phase described by this equation:
SO2(g) + H2O(l) ⇌ H2SO3(aq)
where KH = 1.24 mol/(L atm). (Henry's Law equation can be found in Section 11.3.) Then, using the calculated [H2SO3] as its equilibrium concentration, we have to think about where a pH value can come from. (Hint: Ka values can be found in Appendix H.)
21.64. Therapeutic imaging or cancer treatment (as seen in Problem 21.57) is not the only source of iodine-131 exposure; humans and animals were also exposed to I-131 after nuclear explosions. Large doses of this radioactive isotope have been linked to cancer, especially in children. To counteract this radioactive isotope's buildup in the thyroid gland (and cancer production), potassium iodide tablets were given out to residents local to both the Chernobyl disaster in the Soviet Union (now part of Ukraine) and the Fukushima explosions in Japan. The premise is that the non-radioactive iodine will be absorbed by the thyroid gland instead of the radioactive iodine.
Accessibility
The mathematical equations have been converted to a format that is compatible with e-readers.
About the Authors
Nicole C. Bouvier-Brown
I brought this OER project to the attention of my colleagues at Loyola Marymount University because it directly speaks to my interests. My group’s current work involves the development, implementation, and assessment of course content that enhances students' personal connection to the social context of science. Our goal is to raise awareness of social and environmental inequities, empower students to use their scientific expertise to find solutions, and increase student engagement and sense of belonging.
From my earliest memories of taking nature walks with my mom, I remember a sense of wonder as I studied the details of my natural surroundings. Later, when I knew more about the mathematical, physical, chemical, and biological principles that governed my observations, my experiences were only enhanced. I went on to earn a BS in chemistry/biology with an environmental emphasis at St. Mary’s College of California and a PhD in environmental science, policy, and management at UC Berkeley. I am an environmental chemist who loves to view the world through an interdisciplinary lens.
Although I have been in academia my entire career, I come to the world of social justice from my faith (through Catholic Social Teaching) and environmental impacts of human activities (through environmental justice movements). Empowering students is a priority because as a female in the physical sciences, I have experienced discrimination and still battle with imposter syndrome. As a parent, I know the power of education in shaping our confidence and perseverance.
As an educator, I would like to provide that sense of awe from seeing chemistry in everyday life or extraordinary events. This allows students to make direct connections between content in the classroom and life experiences. I am also interested in the interplay between science and policy. My goal is to empower students to take their scientific knowledge and advocate for the health and well-being of others and the environment. I am also facilitating student contributions to the OER resources; these authors know what will resonate with their peers and can identify gaps or confusion within the textbook that authors (who took general chemistry a long time ago) overlook.
This project has been so much fun. As an introvert, I am happy just plugging along modifying this text to introduce new ways of applying basic chemistry principles to relatable situations and making learning more accessible, interactive, and fun!
Saori Shiraki
After spending my early years in Japan, where I was born, I moved to the United States at the age of eight. When I immigrated, I could only say three phrases in English (hello, bye, and thank you). Due to that language barrier, I found that math was the only class I excel at. That started me off on a love of STEM classes. I also loved the logical mindset math and science requires. As I graduated high school, I was unsure what I wanted to major in at college other than NOT Chemistry. However, when I took organic chemistry (the second-year course), I was shocked to find that I loved it. So I decided to be a chemistry major and never looked back.
After receiving my BS in chemistry from University of California, Davis, I went on to UCLA to get my PhD in organic chemistry. Since then, I’ve taught general chemistry, organic chemistry, and biochemistry lab. I enjoy the challenge of making the course most students dread more interesting—and hopefully fun.
Looking back on my experience as an undergraduate, I realized that I had almost exclusively white male professors in chemistry. And it makes me a little sad to think of the enriching experiences I probably missed out on due to the lack of that diversity. And although East Asians are overrepresented in STEM fields, including chemistry, I experienced the challenges that a woman in a male-dominated field posed. That experience has cemented my commitment to make my classes welcoming to everyone, including underrepresented groups. This textbook gives me the opportunity to expand that effort to those students outside of my classes, which makes this project incredibly important to me.
Jonathan Ryan Hunt
I grew up in Nitro, West Virginia, a town of 6,000 in the heart of Appalachia. My father was an accountant, and my admiration of him gave me an early interest in math. I attended Centre College in Danville, Kentucky, and studied chemistry with a minor in mathematics as part of the Brown Fellows Scholarship Program. Trips to eastern Kentucky as part of the program taught me viscerally about the environmental impacts of coal mining and the socioeconomic inequities of the Appalachian region. These experiences provided a focus for my goals in chemistry and life more broadly: (1) to contribute, in some way, toward a greener future; (2) to help, in some way, address those societal inequities; and (3) to be a positive representative for the Appalachian region.
I received my PhD in physical chemistry from the University of Southern California in Los Angeles. Studying chemistry in a highly diverse city like LA made me realize that, while chemistry is for everyone, more work is needed to make the field feel inclusive for all. I’m so grateful for the opportunity to contribute to this work via Chemistry 3e.
I would like to acknowledge that I identify as white, male, gay, and cisgender. I am currently a tenure-track assistant professor of chemistry and biochemistry at Loyola Marymount University in LA, where I primarily teach general chemistry and physical chemistry courses. My research interests include excited-state dynamics of small molecules, particularly those with applications to green chemistry. In my free time, I love climbing and visiting coffee shops with my husband.
Emily A. A. Jarvis
I was born in Fresno, California, and grew up in the Central Valley of California and Southern Ohio before moving to Orange, California, with my parents and three sisters. We spent a lot of time visiting national parks and camping. Sometimes my sisters and I would set up the tent to camp in our backyard just for fun. It was on these backyard camping trips that I first remember “teaching” science—huddled around a flashlight with my sisters and sharing amazing things I had learned from reading books and school about how our eyes perceive color and other fascinating topics. My sisters would beg for more of these “lectures” until late into the night when I would insist it was time to sleep.
I switched to a chemistry major at Pepperdine University upon discovering I loved the topic during my first year general chemistry courses. Two weeks after graduating, I started graduate school in physical chemistry at the University of California, Los Angeles. I joined the group of Emily A. Carter for my doctorate. The research topics in this group ranged from theory development to molecular and solid-state applications of numerically solving the fundamental equation of quantum mechanics (the Schrödinger equation!). Emily’s research group at this time was primarily comprised of postdoctoral scholars—each from a different country around the world—who were men and years older than me. As a 21-year-old woman, I didn’t think I would ever fit in or enjoy my time there socially. Boy, was I wrong! Graduate school ended up being an amazing time of growth for me where I learned how to learn; tackled fascinating but challenging science; and made friends from China, India, Germany, Argentina, Denmark, Sweden, Vietnam, England, and more—all united by our love of science and passion for learning.
After UCLA, I moved to Washington, DC, to work in the U.S. Senate on science policy as a Congressional Science Fellow through the American Chemical Society organized by the American Association for the Advancement of Science. This provided a glimpse of the many ways science is vital to our modern daily life and the multitude of ways that scientists can help inform decision-making. I also worked as a National Research Council Fellow at the National Institute of Standards and Technology and at Kenyon College and Gordon College before moving to Loyola Marymount University.
I am passionate about green chemistry and involved in the Green Chemistry Commitment organized by Beyond Benign. My research employs quantum chemistry calculations to explore molecules and solids that contribute to alternative energy technologies and catalysis. I teach general and physical chemistry as well as wine chemistry and in the master’s program for entrepreneurship and sustainable innovation in the College of Business. In my free time, I love reading; painting; running, biking, and swimming in pools and open water; and spending time with my family, friends, and church community.
Student Contributors
We would like to thank our student contributors:
- Olivia Kelleher, Chemistry Major at LMU, Class of 2025
- Paula Millan, Biology Major at LMU, Class of 2026
- Amanda Blumberg, Environmental Science Major at LMU, Class of 2028
Reviewers
- Jasmine Bryant, Director of the Center for Teaching, Learning, and Outreach, The California Institute of Technology
- Shuai Sun, Assistant Teaching Professor, The University of Kansas
- Irv Levy, Chemistry Teacher, Pingree School
- Lisa Sharpe Elles, Associate Teaching Professor of Chemistry, The University of Kansas
We would also like to acknowledge all of the previous authors and reviewers of Chemistry 2e.
Media Attributions
- Omowunmi Sadik Speaker at Innovation Day 2010 © Science History Institute is licensed under a CC BY-SA (Attribution ShareAlike) license
- Eunice Foote & the Oil Barons Cartoon © Chris Slane is licensed under a CC BY-SA (Attribution ShareAlike) license
- Excerpt from a summary of Foote's paper that was reported by David A. Wells in the 1857 volume of his Annual of Scientific Discovery ↵
- Natural Gas. U.S. Energy Information Administration. ↵
