Chapter 12. The Respiratory System
12.4 Gas Exchange
Learning Objectives
By the end of this section, you will be able to:
- state the main unit used (partial pressure) to express the amount of a specific gas in a mixture of gases;
- list the typical atmospheric, alveolar, arterial, and venous blood PO2 and PCO2 values;
- describe the partial pressure gradients for and direction of diffusion for O2 and CO2 at the levels of the alveoli and the tissues;
- compare external respiration and internal respiration;
- define perfusion; and
- explain how the body adapts when ventilation and perfusion are mismatched.
The main function of the respiratory system is to perform gas exchange. Via gas exchange, we acquire the oxygen needed for cellular respiration and we eliminate the carbon dioxide produced in cellular respiration.
Pulmonary ventilation provides air to the alveoli for gas exchange to occur. At the respiratory membrane, where the alveolar and capillary walls meet, gases diffuse across the membranes, with oxygen entering the bloodstream from the alveoli and carbon dioxide exiting the bloodstream into the alveoli.
In order to understand the mechanisms of gas exchange in the lung, it is important to understand the underlying principles of gases and their behavior. In addition to Boyle’s law, several other gas laws help to describe the behavior of gases.
Gas Laws and Air Composition
Gas molecules exert pressure on the surfaces with which they are in contact. In natural systems, gases are normally present as a mixture of different types of molecules. For example, the atmosphere consists of oxygen, nitrogen, carbon dioxide, and other gaseous molecules, and this gaseous mixture exerts a certain pressure referred to as atmospheric pressure (Table 12.1).
Partial pressure (Px) is the pressure of a single type of gas in a mixture of gases. For example, in the atmosphere, oxygen exerts a partial pressure, represented as PO2. Nitrogen exerts another partial pressure, represented as PN2, independent of the partial pressure of oxygen (Figure 12.4.1). Total pressure is the sum of all the partial pressures of a gaseous mixture. Dalton’s law describes the behavior of nonreactive gases in a gaseous mixture and states that a specific gas type in a mixture exerts its own pressure; thus, the total pressure exerted by a mixture of gases is the sum of the partial pressures of the gases in the mixture.
| Gas | Percentage in atmosphere (sea level) | Partial pressure
(mm Hg) in atmosphere (sea level)
|
Percentage in alveoli | Partial pressure
(mm Hg) in alveoli
|
|---|---|---|---|---|
| Nitrogen (N2) | 78.6 | 597 | 74.9 | 569 |
| Oxygen (O2) | 20.9 | 159 | 13.7 | 104 |
| Carbon dioxide (CO2) | 0.04 | 0.3 | 5.2 | 40 |
| Water (H2O) | 0.46 | 3.7 | 6.2 | 47 |
| Total | 100 | 760 | 100% | 760 |
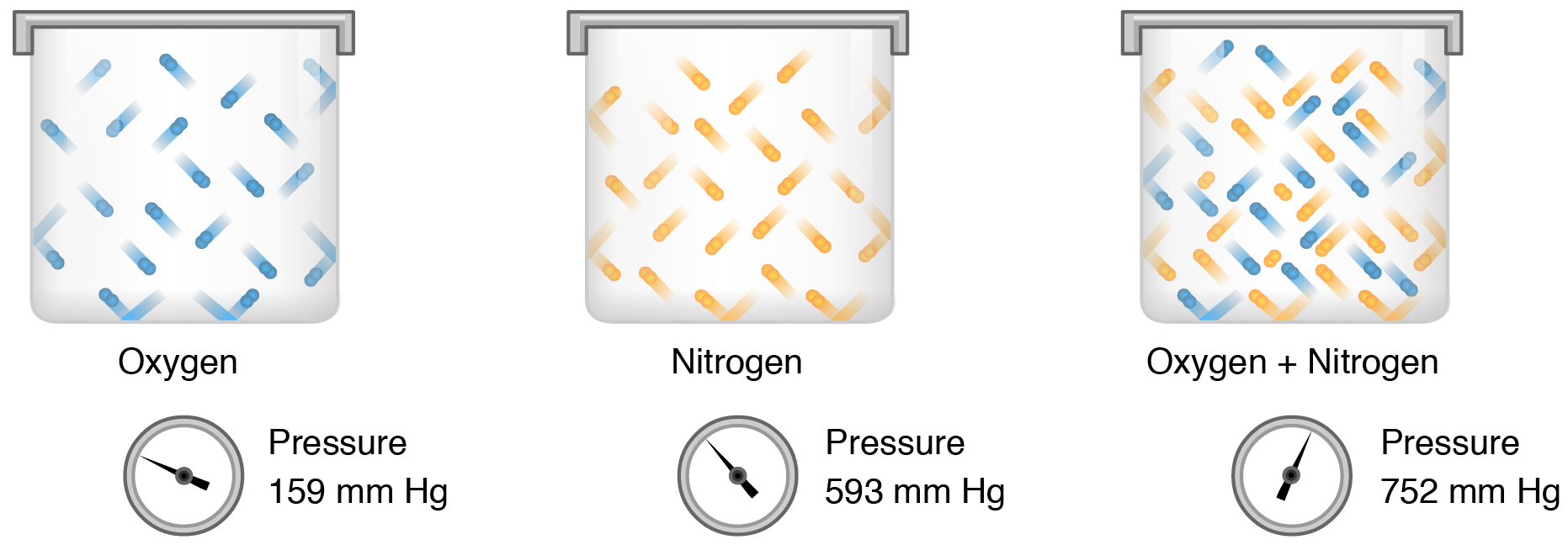
Partial pressure is extremely important in predicting the movement of gases. Recall that gases tend to equalize their pressure in two regions that are connected. A gas will move from an area where its partial pressure is higher to an area where its partial pressure is lower. In addition, the greater the partial pressure difference between the two areas, the more rapid the movement.
Solubility of Gases in Liquids
Henry’s law describes the behavior of gases when they come into contact with a liquid, such as blood. Henry’s law states that the concentration of gas in a liquid is directly proportional to the solubility and partial pressure of that gas. The greater the partial pressure of the gas, the greater the number of gas molecules that will dissolve in the liquid. The concentration of the gas in a liquid is also dependent on the solubility of the gas in the liquid. For example, although nitrogen is present in the atmosphere, very little nitrogen dissolves into the blood, because the solubility of nitrogen in blood is very low. Gas molecules establish an equilibrium between those molecules dissolved in liquid and those in air.
The composition of air in the atmosphere and in the alveoli differs. In both cases, the relative concentrations of gases, from greatest to lowest concentrations, are as follows:
nitrogen > oxygen > water vapor > carbon dioxide
The amount of water vapor present in alveolar air is greater than that in atmospheric air (Table 12.1). Recall that the respiratory system works to humidify incoming air, thereby causing the air present in the alveoli to have a greater amount of water vapor than atmospheric air. In addition, alveolar air contains a greater amount of carbon dioxide and less oxygen than atmospheric air. This is no surprise, as gas exchange removes oxygen from and adds carbon dioxide to alveolar air. Both deep and forced breathing cause the alveolar air composition to be changed more rapidly than during quiet breathing. As a result, the partial pressures of oxygen and carbon dioxide change, affecting the diffusion process that moves these materials across the membrane. This will cause oxygen to enter and carbon dioxide to leave the blood more quickly.
Ventilation and Perfusion
Two important aspects of gas exchange in the lung are ventilation and pulmonary perfusion. As discussed in the previous section, ventilation is the movement of air into and out of the lungs. Perfusion is the flow of blood through a tissue or organ. For gas exchange to be sufficient, ventilation of the alveoli needs to be matched with perfusion of the lungs. However, factors such as regional gravity effects on blood, blocked alveolar ducts, or disease can cause ventilation and perfusion to be imbalanced.
The body responds to a ventilation-perfusion mismatch by changing the diameters of the bronchioles and of the pulmonary arterioles supplying the pulmonary capillaries. In short, PO2 levels regulate arteriolar diameter and PCO2 levels regulate bronchiolar diameter (Figure 12.4.2 and Figure 12.4.3).
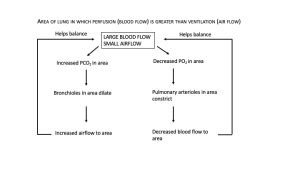
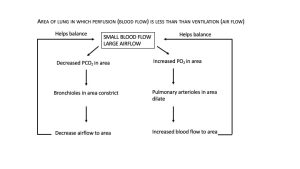
Gas Exchange
Gas exchange occurs at two sites in the body:
- In the lungs, where oxygen diffuses from the alveoli into the blood and carbon dioxide diffuses from the blood into the alveoli at the respiratory membrane; this is termed external respiration.
- At the tissues, where oxygen diffuses from the blood into the tissues and carbon dioxide diffuses from the tissues into the blood; this is termed internal respiration.
As mentioned above, the actual exchange of gases occurs due to simple diffusion. Energy is not required to move oxygen or carbon dioxide across membranes. Instead, these gases follow pressure gradients that allow them to diffuse.
External Respiration
The anatomy of the lung maximizes the diffusion of gases.
External respiration is the exchange of gases between the alveoli of the lungs and the blood in the pulmonary capillaries. The pulmonary arteries carry deoxygenated blood into the lungs from the heart, where they branch into pulmonary arterioles and eventually the pulmonary capillaries. The walls of the capillaries and the walls of the alveoli form the respiratory membrane with (Figure 12.4.4). The respiratory membrane is highly permeable to gases, as the alveolar and capillary membranes are very thin and there is a large surface area throughout the lungs.
As the blood flows through the pulmonary capillaries, gas exchange occurs. Although a small amount of the oxygen is able to dissolve directly into plasma from the alveoli, most of the oxygen is picked up by erythrocytes (red blood cells) and binds to a protein called hemoglobin, a process described in the next section. Oxygenated hemoglobin is red, causing the overall appearance of bright red oxygenated blood, which returns to the heart through the pulmonary veins. Carbon dioxide diffuses in the opposite direction of oxygen, from the blood to the alveoli.
External respiration occurs as a function of partial pressure differences in oxygen and carbon dioxide between the alveoli and the blood in the pulmonary capillaries.
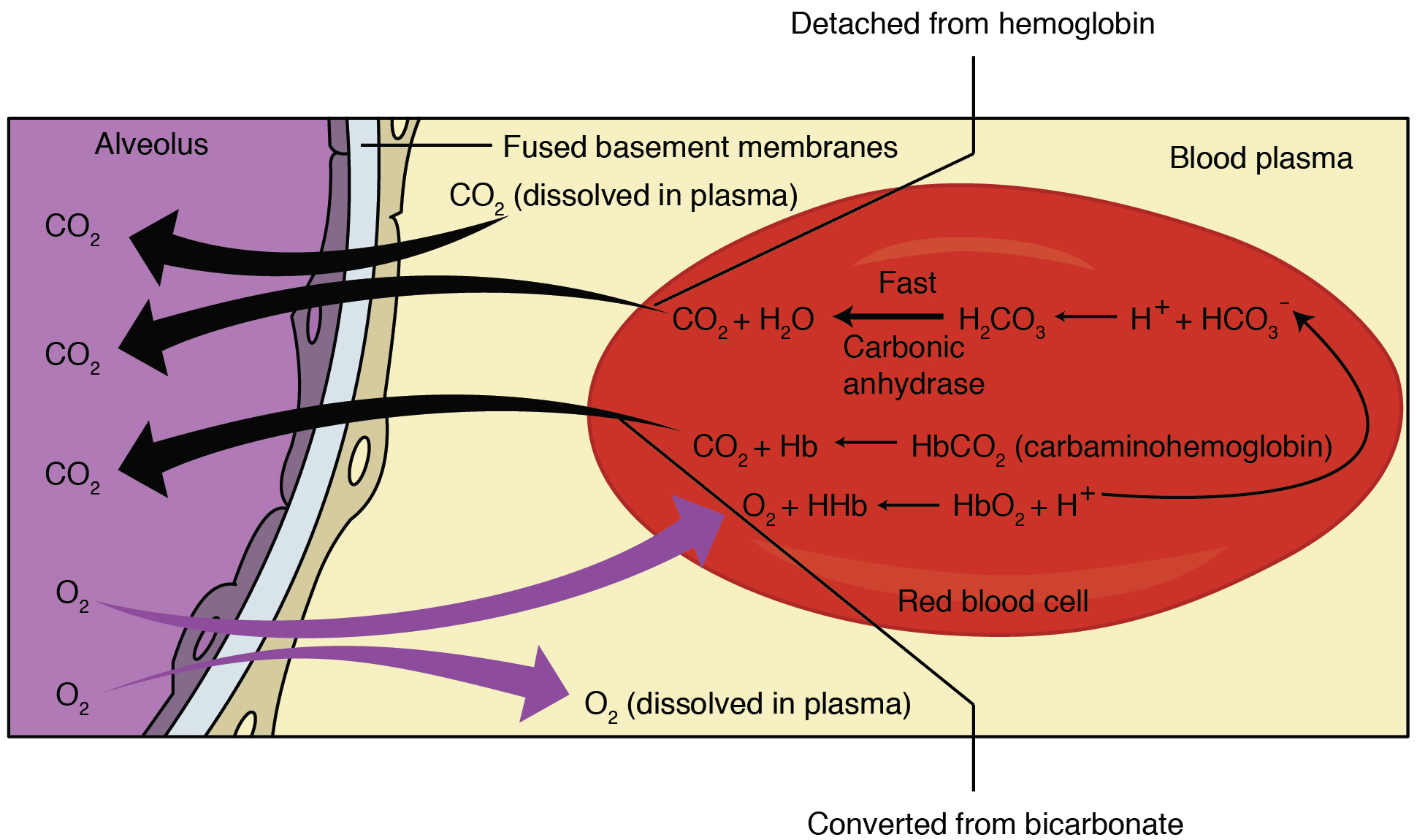
Diffusion of Oxygen from the Alveoli into the Pulmonary Capillaries
Although the solubility of oxygen in blood is not high, there is a drastic difference between the PO2 in the alveoli and the PO2 in the venous blood entering the pulmonary capillaries: the PO2 in the alveoli is about 104 mmHg, whereas PO2 in the venous blood entering the pulmonary capillaries is about 40 mmHg. This large difference in PO2 results in a large PO2 gradient that causes oxygen to rapidly cross the respiratory membrane from the alveoli into the blood.
Diffusion of Carbon Dioxide from the Pulmonary Capillaries into the Alveoli
The PCO2 is also different between the alveolar air and the venous blood entering the pulmonary capillaries: the PCO2 in the venous blood entering the pulmonary capillaries is about 45 mmHg, whereas its partial pressure in the alveoli is about 40 mm Hg. Due to this gradient, carbon dioxide diffuses from the blood into the alveoli.
Although the PCO2 gradient is smaller than that of oxygen (just a 5 mm Hg difference in PCO2 values), the solubility of carbon dioxide is much greater than that of oxygen—by a factor of about 20—in both blood and alveolar fluids. As a result, the relative concentrations of oxygen and carbon dioxide that diffuse across the respiratory membrane are similar.
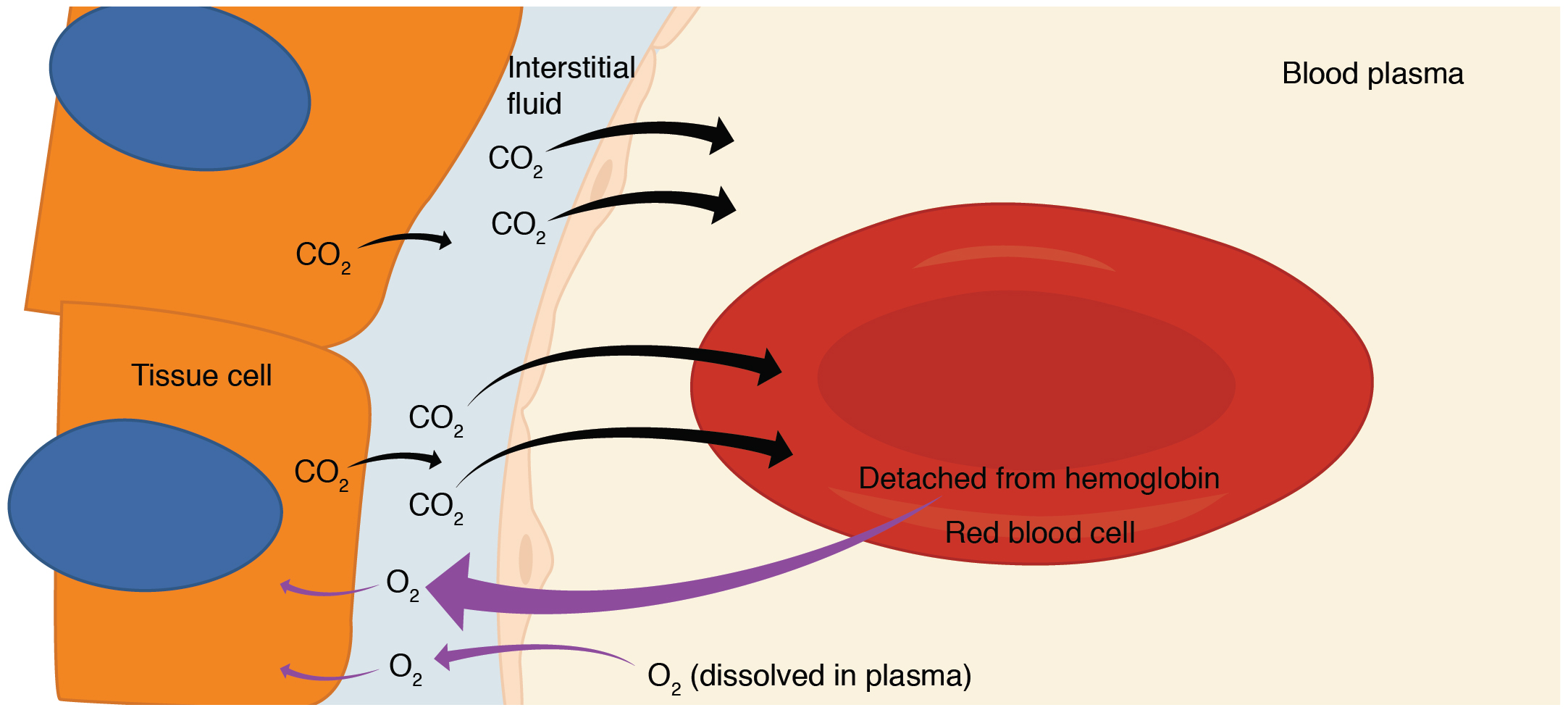
Internal Respiration
Internal respiration is gas exchange that occurs at the level of body tissues (Figure 12.4.5). Similar to external respiration, internal respiration also occurs as simple diffusion due to partial pressure gradient. However, the partial pressure gradients are opposite of those present at the respiratory membrane.
Diffusion of Oxygen from the Systemic Capillaries into the Tissues
The PO2 in tissues is low, about 40 mmHg*, because oxygen is continuously used for cellular respiration. In contrast, the PO2 in the blood is about 100 mm Hg. This creates a pressure gradient that causes oxygen to dissociate from hemoglobin, diffuse out of the blood, and enter the tissue.
*Note that during exercise, the PO2 of exercising muscle tissue is lower since more oxygen is being used to meet the ATP demands of exercising tissue; therefore the gradient for PO2 is larger in this situation and more oxygen will diffuse from the blood into the tissues.
Regardless of the situation (rest or exercise), diffusion of oxygen continues until the PO2 values of the blood and tissues are equal. Therefore, venous blood leaving tissues at rest has a PO2 of about 40 mmHg.
Diffusion of Carbon Dioxide from the Tissues into the Systemic Capillaries
Since cellular respiration continuously produces carbon dioxide, the PCO2 is higher in the tissues than it is in the blood: the PCO2 of tissues is about 45 mmHg and that of the arterial blood entering tissues is about 40 mmHg. This gradient drives carbon dioxide to diffuse out of the tissues and into the blood.
Diffusion of carbon dioxide continues until the PCO2 values of the blood and tissues are equal. Therefore, venous blood leaving tissues at rest has a PCO2 of about 45 mmHg.
Venous blood is then transported to the right side of the heart and then pumped to back the lungs to be oxygenated once again during external respiration.
Everyday Connection – Hyperbaric Chamber Treatment
A type of device used in some areas of medicine that exploits the behavior of gases is hyperbaric chamber treatment. A hyperbaric chamber is a unit that can be sealed and expose a patient to either 100% oxygen with increased pressure or a mixture of gases that includes a higher concentration of oxygen than normal atmospheric air, also at a higher partial pressure than the atmosphere. There are two major types of chambers: monoplace and multiplace. Monoplace chambers are typically for one patient, and the staff tending to the patient observes the patient from outside of the chamber (Figure 12.4.6). Some facilities have special monoplace hyperbaric chambers that allow multiple patients to be treated at once, usually in a sitting or reclining position, to help ease feelings of isolation or claustrophobia. Multiplace chambers are large enough for multiple patients to be treated at one time, and the staff attending these patients is present inside the chamber. In a multiplace chamber, patients are often treated with air via a mask or hood, and the chamber is pressurized.

Hyperbaric chamber treatment is based on the behavior of gases. As you recall, gases move from a region of higher partial pressure to a region of lower partial pressure. In a hyperbaric chamber, the atmospheric pressure is increased, causing a greater amount of oxygen than normal to diffuse into the bloodstream of the patient. Hyperbaric chamber therapy is used to treat a variety of medical problems, such as wound and graft healing, anaerobic bacterial infections, and carbon monoxide poisoning. Exposure to and poisoning by carbon monoxide is difficult to reverse, because hemoglobin’s affinity for carbon monoxide is much stronger than its affinity for oxygen, causing carbon monoxide to replace oxygen in the blood. Hyperbaric chamber therapy can treat carbon monoxide poisoning, because the increased atmospheric pressure causes more oxygen to diffuse into the bloodstream. At this increased pressure and increased concentration of oxygen, carbon monoxide is displaced from hemoglobin. Another example is the treatment of anaerobic bacterial infections, which are caused by bacteria that cannot or prefer not to live in the presence of oxygen. An increase in blood and tissue levels of oxygen helps to kill the anaerobic bacteria that are responsible for the infection, as oxygen is toxic to anaerobic bacteria. For wounds and grafts, the chamber stimulates the healing process by increasing energy production needed for repair. Increasing oxygen transport allows cells to ramp up cellular respiration and thus ATP production, the energy needed to build new structures.
Section Review
The behavior of gases can be explained by the principles of Dalton’s law and Henry’s law, both of which describe aspects of gas exchange. Dalton’s law states that each specific gas in a mixture of gases exerts force (its partial pressure) independently of the other gases in the mixture. Henry’s law states that the amount of a specific gas that dissolves in a liquid is a function of its partial pressure. The greater the partial pressure of a gas, the more of that gas will dissolve in a liquid. Gas molecules move down a partial pressure gradient; in other words, gas diffuses from a region of high partial pressure to a region of lower partial pressure.
External respiration refers to gas exchange that occurs in the alveoli, whereas internal respiration refers to gas exchange that occurs in the tissue. Both occur via diffusion down partial pressure gradients.
Ventilation is the process that moves air into and out of the alveoli, and perfusion is the flow of blood through a tissue or organ. For gas exchange to be sufficient, ventilation of the alveoli needs to be matched with perfusion of the lungs.
Review Questions
Critical Thinking Questions
Glossary
- Dalton’s law
- statement of the principle that a specific gas type in a mixture exerts its own pressure
- external respiration
- gas exchange that occurs in the alveoli
- Henry’s law
- statement of the principle that the concentration of gas in a liquid is directly proportional to the solubility and partial pressure of that gas
- internal respiration
- gas exchange that occurs at the level of body tissues
- partial pressure
- force exerted by each gas in a mixture of gases
- ventilation
- movement of air into and out of the lungs; consists of inspiration and expiration
- perfusion
- the flow of blood to a tissue or to an organ; supplying tissues with blood
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
