Chapter 12. The Respiratory System
12.5 Transport of Gases
Learning Objectives
By the end of this section, you will be able to:
- describe the two ways in which oxygen is carried in the blood;
- describe the structure of hemoglobin;
- explain how changes in temperature and in pH affect hemoglobin-oxygen affinity;
- compare and contrast fetal and adult hemoglobin; and
- describe the three ways in which carbon dioxide is transported in the blood.
As mentioned in the previous section, the function of gas exchange is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialized transport system for the majority of the gas molecules to be moved between the lungs and other tissues.
Oxygen Transport in the Blood
Even though oxygen is transported via the blood, you may recall that oxygen is not very soluble in liquids. A small amount of oxygen does dissolve in the plasma and is transported there, but only about 1.5% of the total amount. The remaining 98.5% of the oxygen in the blood is transported in erythrocytes bound to hemoglobin molecules.
Hemoglobin is one of two main respiratory pigments in the body; the other respiratory pigment is myoglobin in muscle tissue. “Respiratory pigment” refers to the fact that hemoglobin transports respiratory gases and is pigmented (Figure 12.5.1). The detailed structure of hemoglobin is described in Chapter 11.3. Recall that each molecule of hemoglobin consists of four globin proteins, each containing a heme group. The heme group is the portion of hemoglobin that contains iron (Fe2+), and therefore one hemoglobin molecule is capable of carrying up to four molecules of oxygen. As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by hemoglobin. The following reversible chemical reaction describes the production of the final product, oxyhemoglobin (Hb–O2), which is formed when oxygen binds to hemoglobin:
Oxyhemoglobin is a bright red-colored molecule that contributes to the bright red color of oxygenated blood. This is in contract to deoxyhemoglobin, which is purplish in color and which give dexoygenated blood a darker red/maroon color.
There are multiple factors involved in how readily heme binds to and dissociates from oxygen, which will be discussed in the subsequent sections.
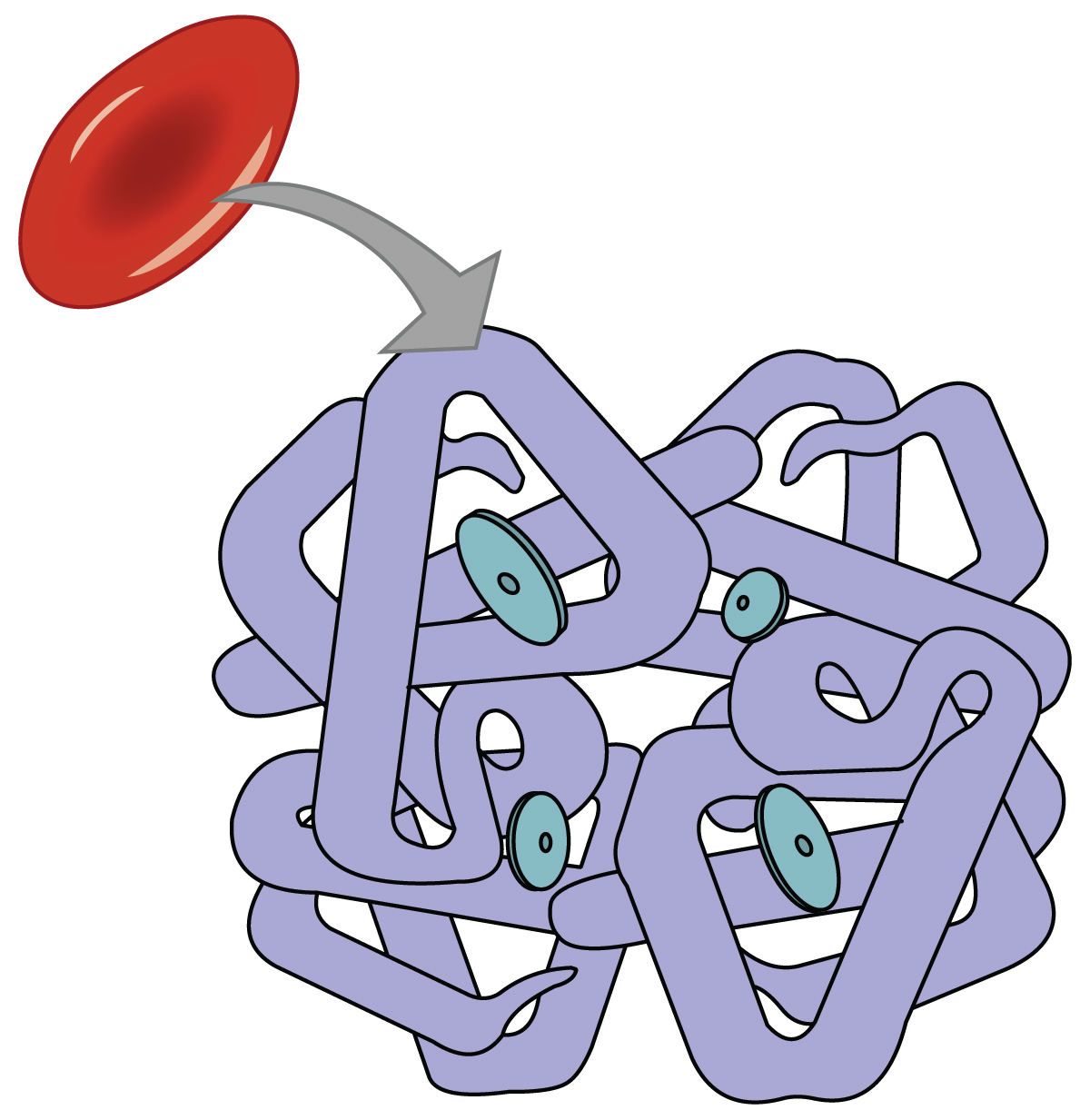
Hemoglobin-Oxygen Binding
Oxygen binds to hemoglobin in a pattern called cooperative binding: binding of the first oxygen molecule causes a conformational change in hemoglobin that allows the second molecule of oxygen to bind more readily. And, as each subsequent oxygen molecule binds, the binding of the next molecule is facilitated until all four heme sites are occupied by oxygen. The opposite occurs as well: after the first oxygen molecule dissociates and is “dropped off” at the tissues, the next oxygen molecule dissociates more readily.
When all four heme sites are occupied, the hemoglobin is said to be saturated. When one to three heme sites are occupied, the hemoglobin is said to be partially saturated. When considering the blood as a whole, the percent of the available heme units that are bound to oxygen at a given time is called hemoglobin saturation. Hemoglobin saturation of 100% means that every heme unit in all of the hemoglobin molecules in the body is bound to oxygen. In a healthy individual with normal hemoglobin levels, hemoglobin saturation generally ranges from 95% to 99%.
Relationship Between PO2 and Hemoglobin Saturation
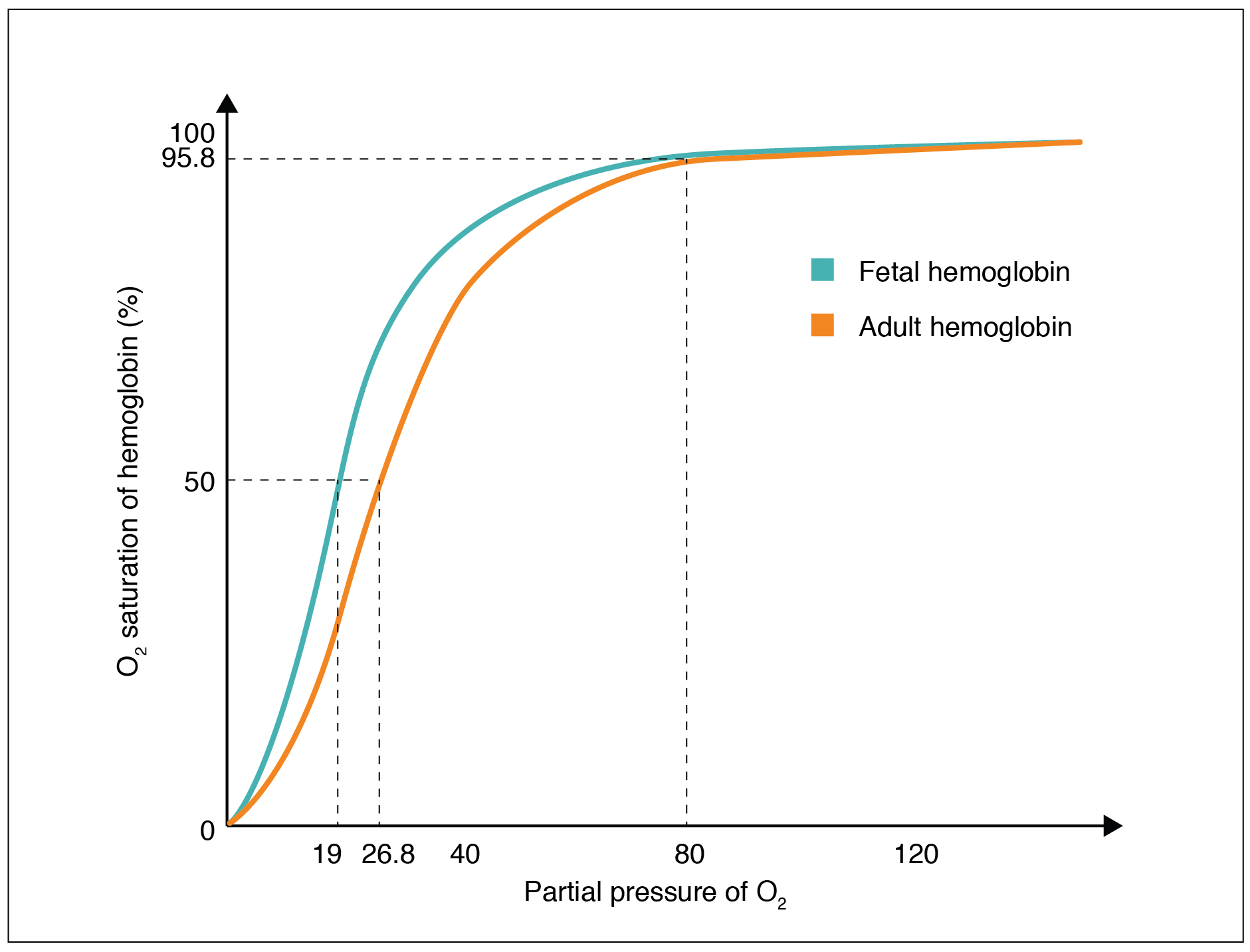
Local PO2 (for example PO2 in the lung environment or in the systemic tissue environment) is an important factor that determines the binding of oxygen to and dissociation of oxygen from hemoglobin. An oxygen–hemoglobin dissociation curve is a graph that describes the relationship of local PO2 to the percent saturation of hemoglobin with oxygen (Figure 12.5.2).
From an oxygen-hemoglobin dissociation curve, we can gain insight regarding hemoglobin-oxygen affinity. Hemoglobin-oxygen affinity refers to the ability of hemoglobin to bind oxygen. Due to the cooperative binding described above, the affinity of an oxygen molecule for hemoglobin increases as more oxygen molecules are bound. Therefore, in the oxygen–hemoglobin saturation curve, as the PO2 increases, a proportionately greater number of oxygen molecules are bound by hemoglobin. Not surprisingly, the oxygen–hemoglobin saturation/dissociation curve also shows that the lower the PO2, the lower the percent saturation of hemoglobin with oxygen. As a result, the PO2 plays a major role in determining the degree of binding of oxygen to heme at the site of the respiratory membrane, as well as the degree of dissociation of oxygen from heme at the site of body tissues.
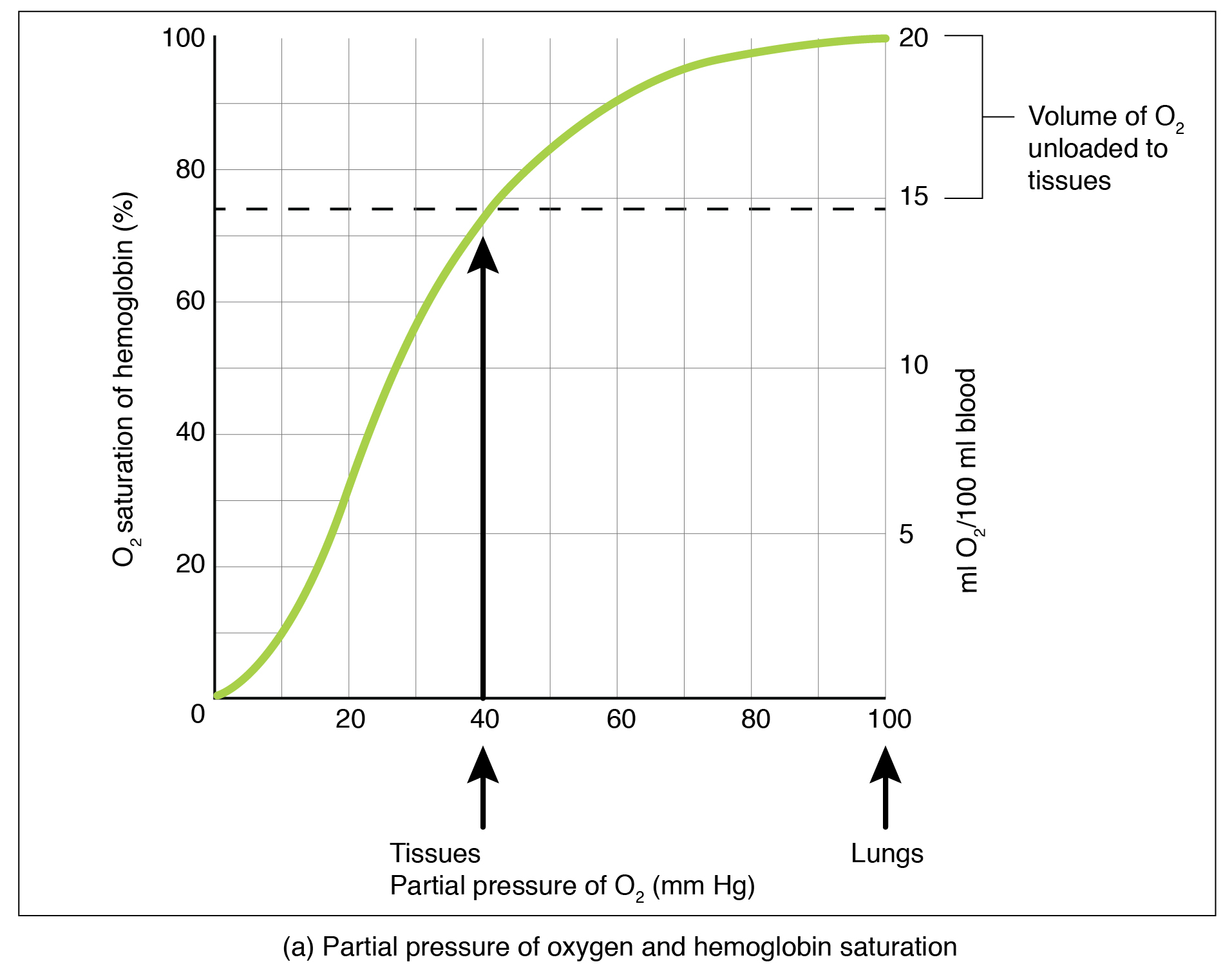
The conditions in which hemoglobin loads oxygen and dissociates from (unloads) oxygen, illustrated in the oxygen–hemoglobin saturation/dissociation curve, are adaptive for delivering different amounts of oxygen to different tissues throughout the body depending on their metabolism. Highly active tissues, such as exercising muscle, rapidly use oxygen to produce ATP, lowering the partial pressure of oxygen in the tissue to about 20 mm Hg. The partial pressure of oxygen inside capillaries is about 100 mm Hg, so the difference between the two becomes quite high, about 80 mm Hg. As a result, a greater number of oxygen molecules dissociate from hemoglobin and enter the tissues.
The reverse is true of tissues, such as adipose (body fat), which have lower metabolic rates. Because less oxygen is used by these cells, the partial pressure of oxygen within such tissues remains relatively high, resulting in fewer oxygen molecules dissociating from hemoglobin and entering the tissue interstitial fluid. Although venous blood is said to be deoxygenated, some oxygen is still bound to hemoglobin in its red blood cells. The amount of oxygen in venous blood after leaving the tissues is called the venous reserve, which can be used when tissues suddenly demand more oxygen.
Additional Factors Affecting Hemoglobin-Oxygen Affinity
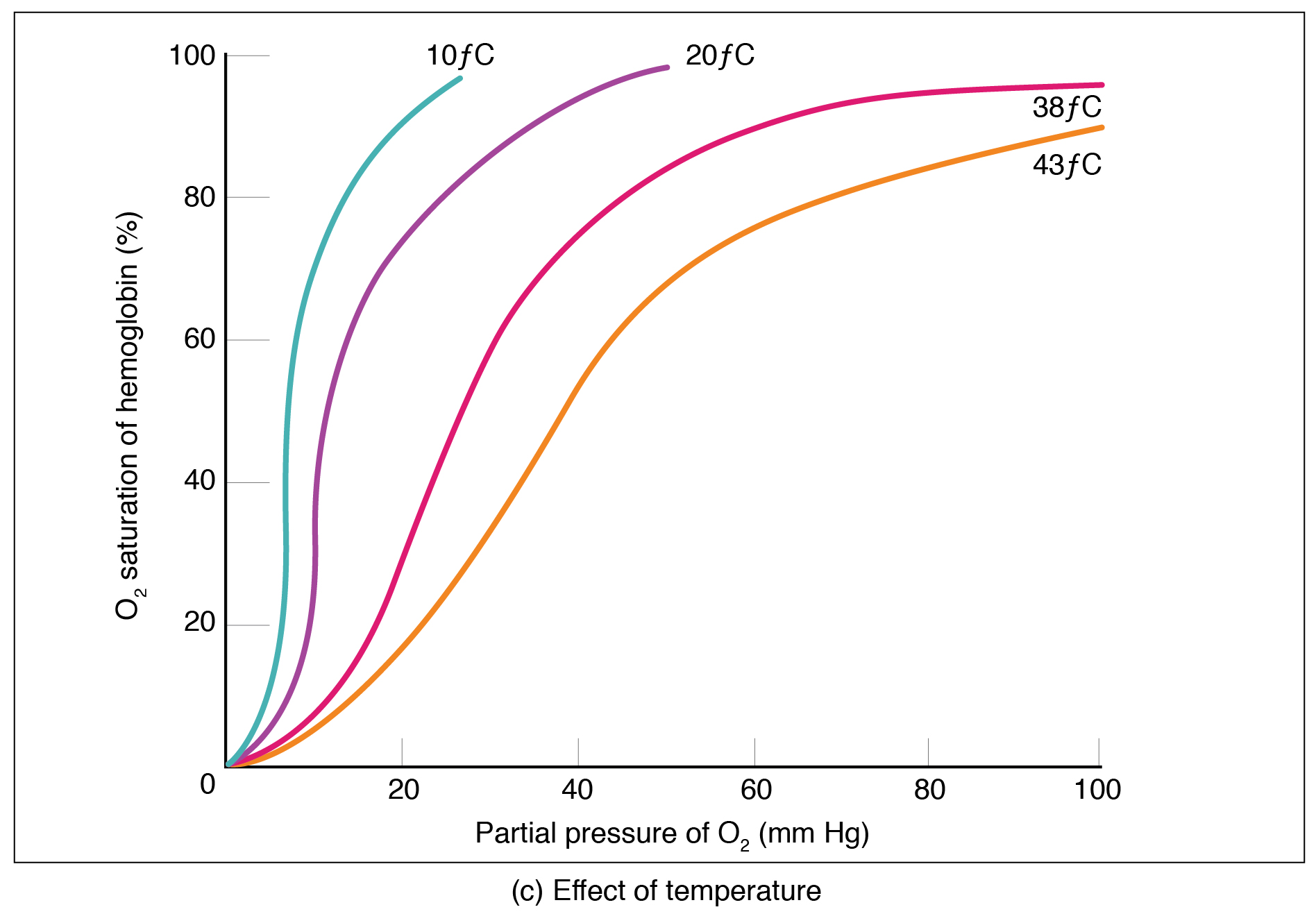
Factors other than PO2 also affect hemoglobin-oxygen affinity. For example, a higher temperature decreases hemoglobin-oxygen affinity, which promotes hemoglobin and oxygen to dissociate faster, whereas a lower temperature increases affinity These differences in affinity at different temperatures are seen in Figure 12.5.2. When comparing the curves at 10C and 20C, for example, it can be seen that at 10C, the hemoglobin is almost 100% saturated at a PO2 of just over 20 mmHg and at 20C, it is not 100% saturdated until just over 40 mmHg.
The chemical 2,3-bisphosphoglycerate (BPG) also affects hemoglobin-oxygen affinity. 1,3-BPG is produced during glycolysis, and certain cells, including erythrocytes, are able to convert this to 2,3-BPG. As 2,3-BPG levels increase, hemoglobin-oxygen affinity decreases. Therefore, the greater the concentration of 2,3-BPG, the more readily oxygen dissociates from hemoglobin, despite its partial pressure.
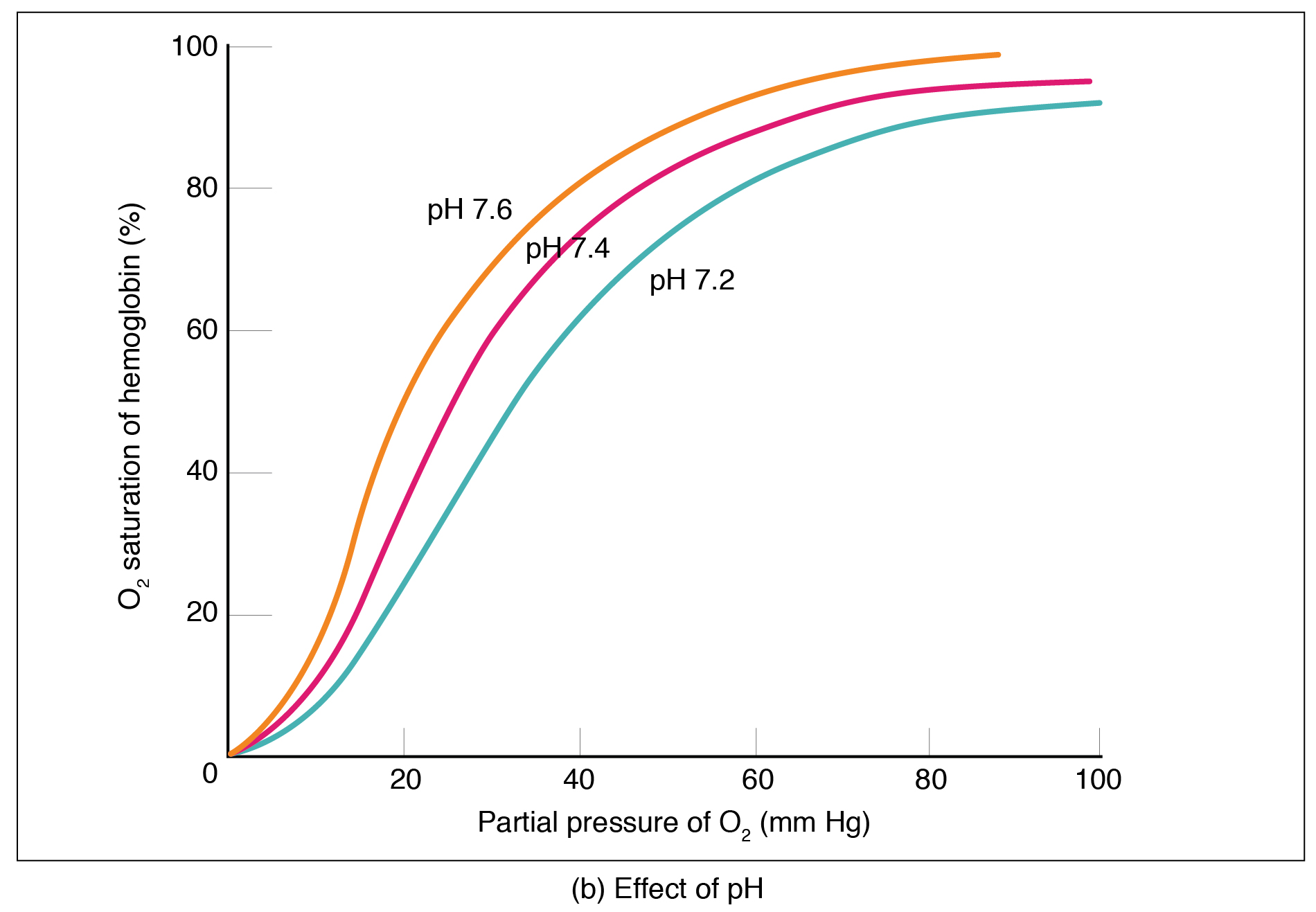
The pH of the blood is another factor that influences hemoglobin-oxygen affinity (Figure 12.5.2). The Bohr effect is a phenomenon that arises from the relationship between pH and oxygen’s affinity for hemoglobin: a lower, more acidic pH lowers hemoglobin-oxygen affinity, in other words it promotes oxygen dissociation from hemoglobin. In contrast, a higher, or more basic, pH increases affinity.
What causes pH changes that result in the Bohr effect? Increased carbon dioxide levels can lead to an increase in H+ concentration, which in turn lowers pH. Furthermore, blood pH may become more acidic when other acidic byproducts of cell metabolism, such as lactic acid, are released into the bloodstream.
Hemoglobin of the Fetus
The fetus has its own circulation with its own erythrocytes; however, it is dependent on the mother for oxygen. Blood is supplied to the fetus by way of the umbilical cord, which is connected to the placenta and separated from maternal blood by the chorion. The mechanism of gas exchange at the chorion is similar to gas exchange at the respiratory membrane. However, the partial pressure of oxygen is lower in the maternal blood in the placenta, at about 35 to 50 mm Hg, than it is in maternal arterial blood. The difference in partial pressures between maternal and fetal blood is not large, as the partial pressure of oxygen in fetal blood at the placenta is about 20 mm Hg. Therefore, there is not as much diffusion of oxygen into the fetal blood supply. The fetus’ hemoglobin overcomes this problem by having a greater affinity for oxygen than maternal hemoglobin (Figure 12.5.3). Both fetal and adult hemoglobin have four polypeptide subunits, but structural differences (different amino acid sequences) between two of the subunits of fetal hemoglobin and adult hemoglobin cause fetal hemoglobin to have a greater affinity for oxygen than adult hemoglobin. Specifically, fetal hemoglobin does not response to BPG as strongly as adult hemoglobin, so therefore has a greater affinity for oxygen.
Carbon Dioxide Transport in the Blood
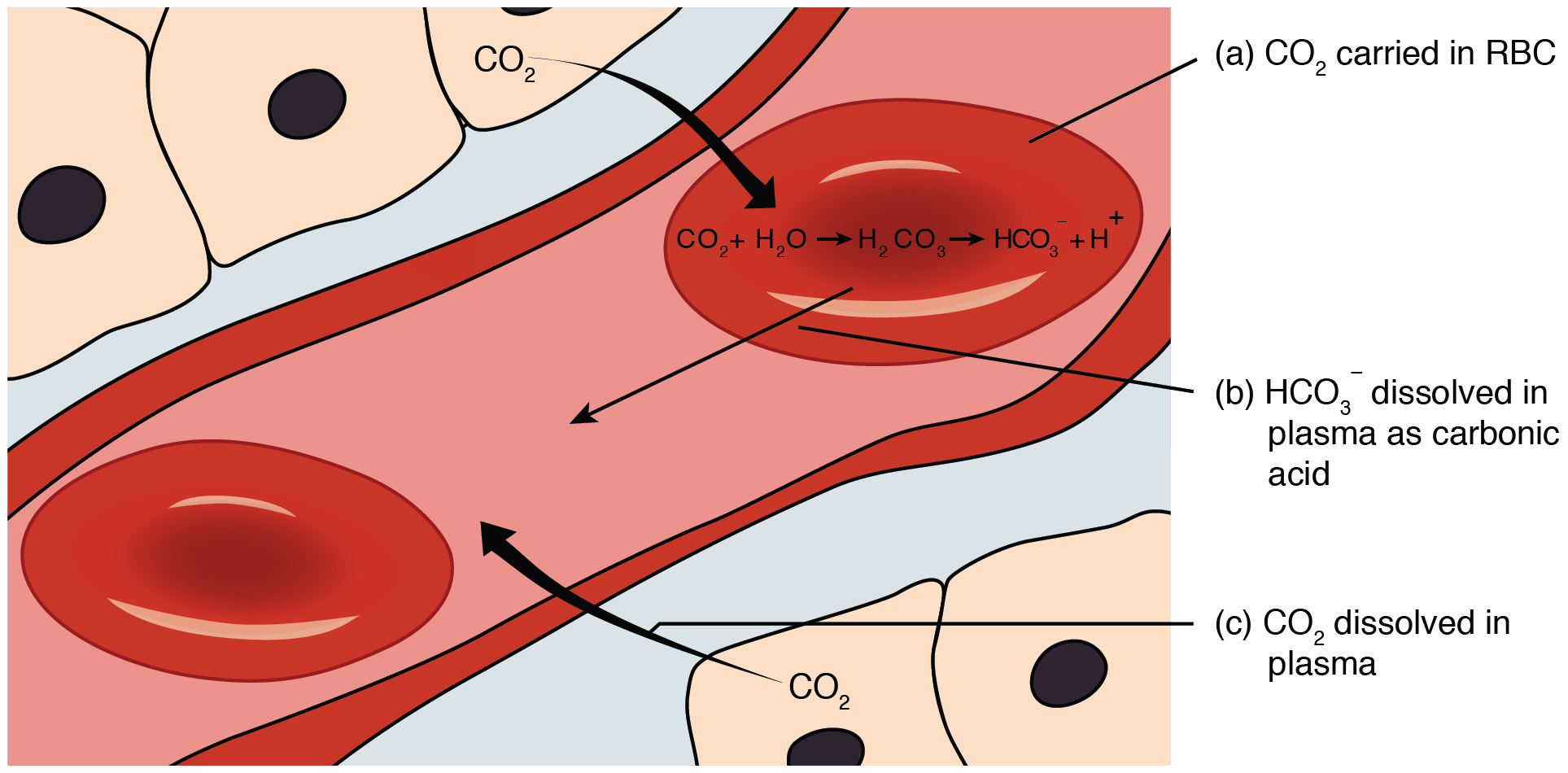
Carbon dioxide is transported by three major mechanisms. The first mechanism of carbon dioxide transport is by blood plasma, as some carbon dioxide molecules dissolve in the blood. The second mechanism is transport in the form of bicarbonate (HCO3–), which also dissolves in plasma. The third mechanism of carbon dioxide transport is via hemoglobin (Figure 12.5.4).
Dissolved Carbon Dioxide
Although carbon dioxide is not highly soluble in blood, a small fraction—about 7%—of the carbon dioxide that diffuses into the blood from the tissues dissolves in plasma. The dissolved carbon dioxide then travels in the bloodstream and when the blood reaches the pulmonary capillaries, the dissolved carbon dioxide diffuses across the respiratory membrane into the alveoli, where it is then exhaled during pulmonary ventilation.
Carbon Dioxide Transported as Bicarbonate
A large fraction—about 70%—of the carbon dioxide molecules that diffuse into the blood is transported to the lungs as bicarbonate. Most bicarbonate is produced in erythrocytes after carbon dioxide diffuses into the capillaries, and subsequently into red blood cells. There, carbon dioxide combines with water to form to form carbonic acid (H2CO3), which dissociates into two ions: bicarbonate (HCO3–) and hydrogen (H+). The following formula depicts this reaction:
This reaction occurs spontaneously, but also is sped up by the action of the enzyme carbonic anhydrase (CA) that catalyzes the formation of carbonic acid from carbon dioxide and water. Bicarbonate tends to build up in the erythrocytes, so that there is a greater concentration of bicarbonate in the erythrocytes than in the surrounding blood plasma. As a result, some of the bicarbonate will leave the erythrocytes and move down its concentration gradient into the plasma in exchange for chloride (Cl–) ions. This phenomenon is referred to as the chloride shift and occurs because by exchanging one negative ion for another negative ion, neither the electrical charge of the erythrocytes nor that of the blood is altered.
At the pulmonary capillaries, the chemical reaction that produced bicarbonate (shown above) is reversed, and carbon dioxide and water are the products. Much of the bicarbonate in the plasma re-enters the erythrocytes in exchange for chloride ions. Hydrogen ions and bicarbonate ions join to form carbonic acid, which is converted into carbon dioxide and water by carbonic anhydrase. Carbon dioxide diffuses out of the erythrocytes and into the plasma, where it can further diffuse across the respiratory membrane into the alveoli to be exhaled during pulmonary ventilation.
Carbaminohemoglobin
About 23% of carbon dioxide is bound by hemoglobin and is transported to the lungs. Carbon dioxide does not bind to iron as oxygen does; instead, carbon dioxide binds amino acid moieties on the globin portions of hemoglobin to form carbaminohemoglobin, which forms when hemoglobin and carbon dioxide bind. The following formula depicts this reversible reaction:
Similar to the transport of oxygen by heme, the binding and dissociation of carbon dioxide to and from hemoglobin is dependent on the partial pressure of carbon dioxide. Because carbon dioxide is released from the lungs, blood that leaves the lungs and reaches body tissues has a lower partial pressure of carbon dioxide than is found in the tissues. As a result, carbon dioxide leaves the tissues because of its higher partial pressure, enters the blood, and then moves into red blood cells, binding to hemoglobin. In contrast, in the pulmonary capillaries, the partial pressure of carbon dioxide is high compared to within the alveoli. As a result, carbon dioxide dissociates readily from hemoglobin and diffuses across the respiratory membrane into the air.
In addition to the partial pressure of carbon dioxide, the oxygen saturation of hemoglobin and the partial pressure of oxygen in the blood also influence the affinity of hemoglobin for carbon dioxide. Hemoglobin that is saturated with oxygen does not readily bind carbon dioxide. However, when oxygen is not bound to heme and the partial pressure of oxygen is low, hemoglobin readily binds to carbon dioxide.
Section Review
Oxygen is primarily transported through the blood by hemoglobin in erythrocytes. When all of the heme groups in the blood are bound to oxygen, hemoglobin is considered to be saturated. Hemoglobin is partially saturated when only some heme units are bound to oxygen. An oxygen–hemoglobin dissociation curve is a common way to depict the relationship of how easily oxygen binds to or dissociates from hemoglobin as a function of local PO2. As PO2 increases, the more readily hemoglobin binds to oxygen. Other factors such as temperature, pH, the partial pressure of carbon dioxide, and the concentration of 2,3-BPG can affect hemoglobin-oxygen affinity as well. Fetal hemoglobin has a different structure than adult hemoglobin and is insensitive to the effects of 2.3 BPG, which results in fetal hemoglobin having a greater affinity for oxygen than adult hemoglobin.
Carbon dioxide is transported in blood by three different mechanisms: 7% as dissolved in the plasma, 23% as carbaminohemoglobin, and 70% as bicarbonate. For transportation as bicarbonate, carbon dioxide is combined with water, a spontaneous reaction that is sped up by the enzyme carbonic anhydrase. This combination forms carbonic acid, which spontaneously dissociates into bicarbonate and hydrogen ions. As bicarbonate builds up in erythrocytes, it is moved across the membrane into the plasma in exchange for chloride ions by a mechanism called the chloride shift. At the pulmonary capillaries, bicarbonate re-enters erythrocytes in exchange for chloride ions, and the reaction with carbonic anhydrase is reversed, recreating carbon dioxide and water. Carbon dioxide then diffuses out of the erythrocyte and across the respiratory membrane into the air. An intermediate amount of carbon dioxide binds directly to hemoglobin to form carbaminohemoglobin. The partial pressures of carbon dioxide and oxygen, as well as the oxygen saturation of hemoglobin, influence how readily hemoglobin binds carbon dioxide. The less saturated hemoglobin is and the lower the partial pressure of oxygen in the blood is, the more readily hemoglobin binds to carbon dioxide.
Review Questions
Critical Thinking Questions
Glossary
- Bohr effect
- the relationship between blood pH and oxygen dissociation from hemoglobin; as pH decreases (acidity increases), hemoglobin-oxygen affinity decreases
- carbaminohemoglobin
- bound form of hemoglobin and carbon dioxide
- carbonic anhydrase (CA)
- enzyme that catalyzes the reaction that causes carbon dioxide and water to form carbonic acid
- hemoglobin
- respiratory pigment in blood that transports oxygen
- hemoglobin-oxygen affinity
- the ability of hemoglobin to bind oxygen
- oxygen–hemoglobin dissociation curve
- graph that describes the relationship between partial pressure of oxygen to the binding and % saturation of hemoglobin with oxygen
- venous reserve
- the amount of oxygen in venous blood after leaving the tissues
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
