Chapter 14. Fluid, Electrolyte, and Acid-Base Balance
14.3 Acid-Base Balance
Learning Objectives
By the end of this section, you will be able to:
- list the three main buffer systems in the body;
- identify the most rapid buffer system in the body;
- state the most important buffer in the ECF;
- state the most important buffer in the ICF;
- define compensation;
- explain the how the respiratory system can regulate plasma pH; and
- explain the how the kidneys can regulate plasma pH.
Maintenance of plasma pH within the homeostatic range, 7.35 to 7.45, is essential for healthy physiological function. Specifically, pH affects the functioning of all proteins; for example:
- All enzymes function optimally in a specific pH range; therefore, all metabolic pathways can be affected by pH changes.
- Hemoglobin function is affected by pH: Hb-O2 affinity decreases as pH decreases.
- Membrane proteins are affected by pH because a change in pH could affect the conformation of the protein.
Plasma pH is maintained by the following three mechanisms:
- buffer systems: these are the first to respond to an acid-base disturbance and act within seconds;
- respiratory mechanisms: these are the next to respond to an acid-base disturbance and can have an effect within 1 to 3 minutes;
- renal mechanisms: these are the last to respond to an acid-base disturbance, having an effect between 1 to 3 days, but are the most potent mechanism for correcting an acid-base disturbance.
Acidity of a solution is measured using the pH scale, as shown in Figure 14.3.1. Blood, at 7.35 to 7.45, is slightly alkaline. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow pH range, even in the face of perturbations. A buffer is a chemical system that prevents a drastic change in fluid pH by dampening the change in H+ concentration in the case of excess acid or base. Most commonly, the buffering substance is either a weak acid, which takes up hydroxyl ions (OH–), or a weak base, which takes up H+.

Buffer Systems in the Body
The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical buffers in the blood to make adjustments to pH. The respiratory tract can adjust the blood pH in minutes by changing ventilation rate. The kidneys can adjust blood pH via changing the secretion of H+ and the reabsorption of bicarbonate, but this process takes hours to days to have an effect.
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and the carbonic acid-bicarbonate buffer system.
Protein Buffers in Plasma and Cells
Nearly all proteins can function as buffers. Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. The charged regions of these molecules can bind H+ and OH– ions, and thus function as buffers.
Protein buffers are the main buffer system in the ICF. In the ECF, the plasma proteins contribute to the buffering capability of the blood.
Hemoglobin as a Buffer
Hemoglobin is the principal protein inside of red blood cells and accounts for one-third of the mass of the cell. During the conversion of CO2 into bicarbonate, H+ liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. This buffering helps maintain normal erythrocyte pH.
Phosphate Buffer
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4−), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO42-), which is a weak base. When Na2HPO42- comes into contact with a strong acid, such as HCl, the base picks up a second hydrogen ion to form the weak acid Na2H2PO4− and sodium chloride, NaCl:
When Na2HPO42− (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid reverts back to the weak base and produces water.
Phosphates are important buffers in urine and the ICF. Phosphates are low in concentration in the ECF, so are not important ECF buffers.
Carbonic Acid-Bicarbonate Buffer System
The carbonic acid-bicarbonate buffer system, discussed in the previous section of this chapter and in other chapters, is the most important ECF buffer. It is a mixture of carbonic acid and sodium bicarbonate, a weak base, and it works in similar fashion to the phosphate buffer system.
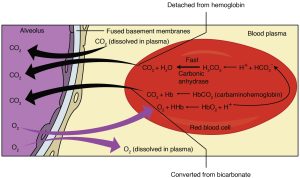
The level of bicarbonate in the blood is controlled via the kidneys, where bicarbonate ions in the renal filtrate are reabsorbed and passed back into the blood. However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body.
Respiratory Regulation of Acid-Base Balance
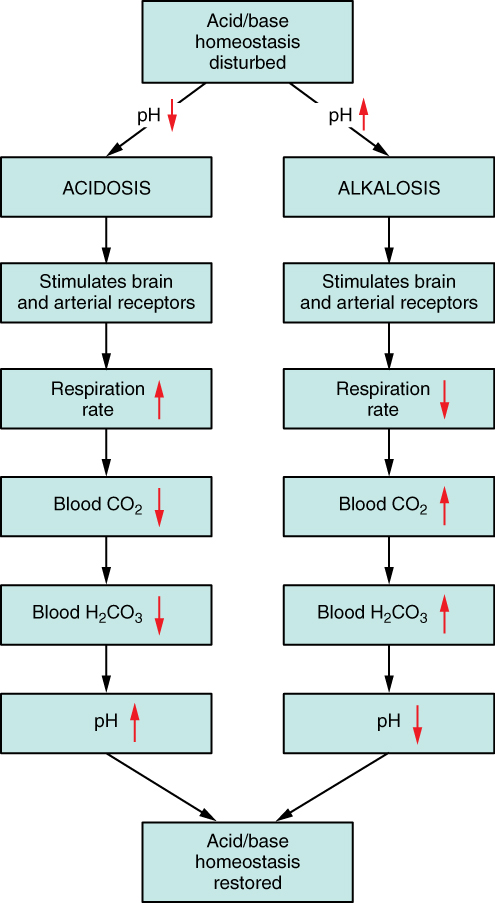
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3−
- hyperventilation -> decreased plasma CO2 -> decreased plasma H+ -> increased plasma pH
- hypoventilation -> increased plasma CO2 -> increased plasma H+ -> decreased plasma pH
Note that hyperventilation and hypoventilation can be the causes acid-base imbalances, or they can be compensations to correct acid base imbalances (Figure 14.3.3.) A compensation when referring to acid-base balance is a physiological response that corrects and acid-base imbalance.
Renal Regulation of Acid-Base Balance
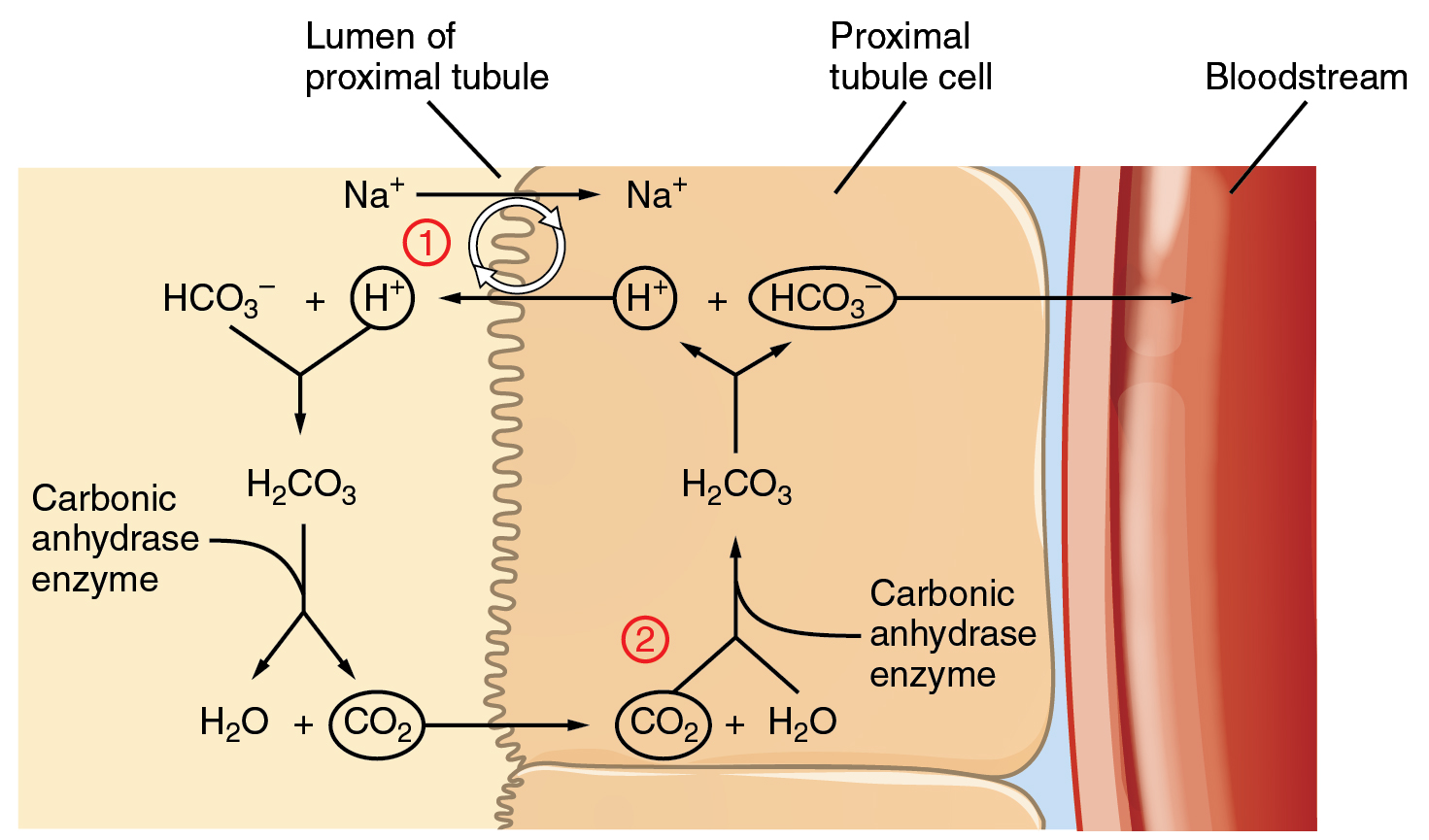
The cells of the PCT reabsorb HCO3– and actively secrete H+ into the filtrate. Bicarbonate ions found in the filtrate are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. The steps involved in supplying bicarbonate ions to the system are seen in Figure 14.3.4 and are summarized below:
- Step 1: In the lumen of the PCT, HCO3– combines with H+ to form carbonic acid.
- Note the source of H+: Na+ ions are reabsorbed from the filtrate in exchange for H+ by an antiport mechanism in the apical membranes of cells lining the renal tubule.
- Carbonic anhydrase catalyzes the dissociation of carbonic acid into CO2 and water, which diffuse across the apical membrane into the cell.
- Step 2: Inside the cell, the reverse reaction occurs to produce HCO3–
- These bicarbonate ions are cotransported with Na+ across the basolateral membrane to the interstitial space around the PCT, and HCO3– moves into the blood (= HCO3– reabsorption.)
- The cotransport of HCO3– with Na+ on the basolateral side is not shown in Figure 14.3.4
With certain acid-base imbalances, renal mechanisms will be the main type of compensations used to correct the imbalance:
- if plasma pH is too low: the kidneys increase HCO3– reabsorption and increase H+ secretion, which results in an increase in plasma pH
- if plasma pH is too high: the kidneys decrease HCO3– reabsorption and decrease H+ secretion, which results in a decrease in plasma pH
Section Review
A variety of buffering systems exist in the body that helps maintain the pH of the plasma within a narrow range—between pH 7.35 and 7.45. A buffer is a substance that prevents drastic changes in fluid pH by absorbing excess hydrogen or hydroxyl ions. Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid.
The carbonic acid-bicarbonate buffer system is the primary buffering system of the ECF, and proteins are the primary buffering system in the ICF. The respiratory and renal systems play major roles in acid-base homeostasis.
Review Questions
Critical Thinking Questions
Glossary
- buffer
- a substance or chemical system that prevents a drastic change in pH of a fluid
- compensation
- a physiological response that corrects an acid-base imbalance
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
