Chapter 14. Fluid, Electrolyte, and Acid-Base Balance
14.4 Disorders of Acid-Base Balance
Learning Objectives
By the end of this section, you will be able to:
- state the homeostatic range for plasma pH;
- state the low and high pH limits for life;
- define physiological acidosis and physiological alkalosis;
- state the main indicator of a respiratory acid-base imbalance;
- state the main indicator of a metabolic acid-base imbalance;
- explain how too low or too high plasma PCO2 can lead to respiratory acid-base imbalances;
- state the three blood parameters considered when diagnosing an acid-base imbalance using an arterial blood gas (ABG) test;
- state the homeostatic ranges for plasma PCO2, and plasma HCO3–;
- state the compensation for each of the following: respiratory acidosis, respiratory alkalosis, metabolic acidosis, metabolic alkalosis; and
- identify the type of acid-base imbalance present in a person by evaluating an ABG test result.
As mentioned previously, arterial blood pH is slightly alkaline, maintained between 7.35 and 7.45.
The pH limits for life are a low of 6.8 and a high of 7.8. Below pH 6.8, severe depression of the CNS leads to coma followed by death. In contrast, overexcitement of the nervous system occurs at a pH above 7.8. This can leads to convulsions, muscle tetanus, and eventual respiratory arrest.
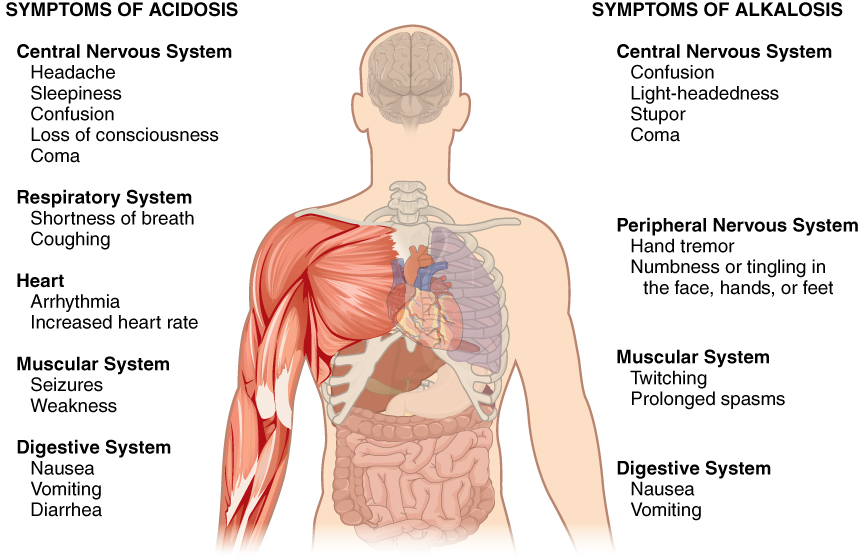
A person who has a blood pH below 7.35 is considered to be in physiological acidosis. Acidosis has several symptoms, including headache and confusion, and the individual can become lethargic and easily fatigued (Figure 14.4.1).
A person who has a blood pH above 7.45 is considered to be in physiological alkalosis. Some symptoms of alkalosis include cognitive impairment (which can progress to unconsciousness), tingling or numbness in the extremities, muscle twitching and spasm, and nausea and vomiting. Both acidosis and alkalosis can be caused by either metabolic or respiratory disorders.
Because the carbonic acid level in the blood is dependent on the level of CO2 in the body and the amount of CO2 gas exhaled through the lungs, the respiratory contribution to acid-base balance is usually discussed in terms of CO2 (rather than of carbonic acid). Remember that a molecule of carbonic acid is lost for every molecule of CO2 exhaled, and a molecule of carbonic acid is formed for every molecule of CO2 retained.
| Distinguishing Blood Parameters | Examples of Causes |
|---|---|
| Respiratory Acidosis (Hypoventilation) | |
|
If uncompensated, PCO2 > 45 mmHg; pH < 7.35 |
|
| Respiratory Alkalosis (Hyperventilation) | |
| If uncompensated, PCO2 < 35 mmHg; pH > 7.45 |
|
| Metabolic Acidosis | |
| If uncompensated, HCO3– < 22 mEq/L; pH < 7.35 |
|
| Metabolic Alkalosis | |
| If uncompensated, HCO3– > 26 mEq/L; pH > 7.45 |
|
Respiratory Acid-Base Imbalances
Respiratory acid-base imbalances are caused by issues with respiratory function that lead to plasma PCO2 values that are too high or too low: out of range PCO2 values are the indicator of a respiratory acid-base imbalance.
Respiratory acidosis is a plasma pH < 7.35 that results from an elevated plasma PCO2 in the blood (Table 14.4). Respiratory acidosis can result from anything that interferes with ventilation or with gas exchange, and is a common cause of acid-base imbalance. causes of respiratory acidosis include such as pneumonia, emphysema, congestive heart failure, paralyzation of respiratory muscles, or depression of brain stem activity.
Respiratory alkalosis is a plasma pH > 7.45 due to a lower than normal plasma PCO2. This condition usually occurs when too much CO2 is exhaled from the lungs, as occurs in hyperventilation. An elevated respiratory rate leading to hyperventilation can be due to extreme emotional upset or fever, infections, or hypoxia. Surprisingly, aspirin overdose—salicylate toxicity—can result in respiratory alkalosis as the body tries to compensate for initial acidosis.
Metabolic Acid-Base Imbalances
Metabolic acid-base imbalances are pH abnormalities caused by any factor except out of range PCO2 values. Indicators of metabolic acid-base imbalances are bicarbonate values out of the normal range (22 to 26 mEq/L).
Metabolic acidosis is a common cause of acid-base imbalance, and is a plasma pH < 7.35 due to too little bicarbonate. Metabolic acidosis can occur with excessive loss of bicarbonate with diarrhea, for example, or with a buildup of acids in the blood. Accumulation of acetic acid can occur with excessive alcohol ingestion, while accumulation of lactic acid can occur with intense exercise or with shock or sepsis. Metabolic acidosis can also arise from ketoacidosis, in which an excess of ketones is present in the blood due to uncontrolled diabetes mellitus or starvation. In each of these situations, the increased metabolism of fats and proteins for fuel leads to the buildup of the acidic ketone bodies.
Metabolic alkalosis is much less common that metabolic acidosis. It occurs when the plasma pH > 7.45 due to elevated bicarbonate.
Vomiting or gastric suctioning causing loss of H+ can result in a proportionate increase in plasma bicarbonate: as stomach acid is lost, H+ from the blood decreases to replace the lost stomach acid.
A transient excess of bicarbonate in the blood can follow ingestion of excessive amounts of bicarbonate, citrate, or antacids to relieve GERD (gastroesophageal reflux, or heartburn). Elevated aldosterone levels can lead to metabolic alkalosis as well: increased renal Na+ reabsorption leads to increased movement of H+ from the plasma into the filtrate. This is because Na+ reabsorption by the DSCT and collecting duct occurs via an antiport: Na+ is moved into the blood and H+ is moved into the filtrate. Other causes of metabolic alkalosis include the use of certain diuretics for hypertension, which can lower blood H+ and proportionately increase blood bicarbonate via a complex mechanism not discussed in this text.
Disorders of Acid-Base Balance – Ketoacidosis
Diabetic acidosis, or diabetic ketoacidosis (DKA), occurs most frequently in people with poorly controlled diabetes mellitus. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy. When acetyl groups break off the fatty acid chains, the acetyl groups then non-enzymatically combine to form ketone bodies, acetoacetic acid, beta-hydroxybutyric acid, and acetone, all of which increase the acidity of the blood.
In this condition, the brain isn’t supplied with enough of its fuel—glucose—to produce all of the ATP it requires to function.
Ketoacidosis can be severe and, if not detected and treated properly, can lead to diabetic coma, which can be fatal. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to drive off CO2 and compensate for the acidosis. Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. Treatment for diabetic coma is ingestion or injection of sugar; its prevention is the proper daily administration of insulin.
External Website
Watch the video below to hear from a person with type I diabetes and their experience with diabetic ketoacidosis. Click here to view “My DKA Experience” by Michelle Lord (January 17, 2018) in a separate tab.
Respiratory and Renal Compensations for Acid-Base Imbalances
Respiratory Compensations for Metabolic Acid-Base Imbalances
Respiratory compensations for metabolic acid-base imbalances involve altering respiratory rate to change plasma PCO2.
Recall that the respiratory system can alter plasma pH rapidly (seconds to minutes) by altering ventilation rate and changing plasma PCO2; this in turn will change plasma H+ by changing the rate of the forward or reverse reaction in the carbonic acid-bicarbonate buffer system:
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3
Renal Compensations for Respiratory Acid-Base Imbalances
Renal compensation for respiratory acid-base imbalances involves altering the renal reabsorption of bicarbonate and secretion of H+ via the PCT and DCT.
Compensation for respiratory acidosis is increased renal bicarbonate reabsorption and increased H+ secretion. These actions result in increased plasma pH.
Compensation for respiratory alkalosis is decreased renal bicarbonate reabsorption and decreased H+ secretion. These actions result in decreased plasma pH.
Diagnosing Acidosis and Alkalosis using ABG Tests
Diagnosing an acid-base imbalance involves measuring arterial blood pH, PCO2, and HCO3. Such a test is called an arterial blood gas, or ABG, test. ABG tests can identify acidosis and alkalosis and whether the imbalance is respiratory or metabolic in nature. In addition, these tests can help determine the extent to which compensatory mechanisms are working.
Table 14.5 lists the imbalances and laboratory ABG results that can be used to classify these conditions.
| Imbalance | Parameter (Normal Range) | ||
|---|---|---|---|
| pH (7.35 to 7.45) | PCO2 (35 to 355 mmHg) | HCO3– (22 to 26 mEq/L) | |
| Metabolic acidosis | ↓ | N;
↓ if compensating |
↓ |
| Respiratory acidosis | ↓ | ↑ | N;
↑ if compensating |
| Metabolic alkalosis | ↑ | N;
↑ if compensating |
↑ |
| Respiratory alkalosis | ↑ | ↓ | N;
↓ if compensation |
How to Solve ABG Problems
The review questions in this section give you a chance to help you practice solving ABG problems. Follow the steps below and use Table 14.5 to help you practice using plasma pH, PCO2, and HCO3- values to determine the cause of an acid-base imbalance in a patient.
- Look at the pH to determine whether the pH is acidotic or alkalotic.
- Check the PCO2 value: is it also out of normal range? If not, then the respiratory system is not causing the imbalance nor compensating for it. If so, is the out of range PCO2 consistent with the pH imbalance?
- Elevated PCO2 can cause an acidosis
- Low PCO2 can cause an alkalosis
- If the out of range PCO2 is not consistent with the pH imbalance, then the altered PCO2 is a compensation, and the imbalance is metabolic in nature.
- Check the bicarbonate value to confirm your findings in steps 2-3: if the condition is not respiratory caused, then the out of range bicarbonate level should confirm either a metabolic acidosis or metabolic alkalosis. If the condition is respiratory caused, and the bicarbonate is out of range, then the altered bicarbonate is a compensation.
Adcomments on problems in which both PCO2 and bicarbonate values are out of range are discussed further below.
If both PCO2 and bicarbonate values are out of the normal range, this indicates that compensation is occurring. These can be challenging to solve, but still follow the rules: try to see if the PCO2 abnormality matches the pH abnormality. If so, it’s a respiratory-caused acid base imbalance that is being compensated for by the kidneys.
Something important to note is that there will be cases where the body has fully compensated for an imbalance and the pH is within the homeostatic range. In these circumstances, it is important to look again at PCO2 and bicarbonate levels to attempt to determine the cause of the imbalance.
Refer to Table 14.5 as you practice, and watch the two videos below for quick mnemonics to help you solve ABGs.
External Websites
Video 2: Click here to view “Acid Base Mnemonic” by baronerock (November 20, 2011) in a different tab. Note that this mechanism is especially useful for ABGs in which pH, PCO2, and bicarbonate values are all out of range.
Section Review
Physiological acidosis and physiological alkalosis describe acid-base imbalances in which a person’s plasma is, respectively, too acidic (pH below 7.35) and too alkaline (pH above 7.45). Each of these conditions can be caused either by metabolic problems related to bicarbonate levels or by respiratory problems related to carbonic acid and CO2 levels. Several compensatory mechanisms allow the body to maintain a normal pH.
Arterial blood gas (ABG) tests measure plasma pH, plasma PCO2, and plasma HCO3– to diagnose the type of and cause of an acid-base imbalance present in a patient.
Review Questions
Critical Thinking Questions
Glossary
- metabolic acidosis
- acid-base imbalance in which a deficiency of bicarbonate causes the blood to be acidotic
- metabolic alkalosis
- acid-base imbalance in which an excess of bicarbonate causes the blood to be alkalotic
- respiratory acidosis
- acid-base imbalance in which an excess of carbonic acid/CO2 causes the blood to be acidotic
- respiratory alkalosis
- acid-base imbalance in which a deficiency of carbonic acid/CO2 causes the blood to be alkalotic
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
