Chapter 16. Metabolism and Nutrition
16.5 Metabolic States of the Body
Learning Objectives
By the end of this section, you will be able to:
- identify the major storage sites (nutrient pools) in the body for carbohydrates, lipids, and proteins, and describe the composition of carbohydrates, lipids, and proteins in each site;
- describe what defines each of the three metabolic states;
- describe the processes that occur during the absorptive state of metabolism;
- describe the processes that occur during the postabsorptive state of metabolism;
- describe the integrated control of blood glucose homeostasis by insulin and glucagon; and
- explain how the body processes glucose when the body is starved of fuel.
You eat periodically throughout the day; however, your organs, especially the brain, need a continuous supply of glucose. How does the body meet this constant demand for energy? Your body processes the food you eat both to use immediately and, importantly, to store as energy for later demands. If there were no method in place to store excess energy, you would need to eat constantly in order to meet energy demands. Distinct mechanisms are in place to facilitate energy storage, and to make stored energy available during times of fasting and starvation.
Nutrient Pools
Throughout the day, the body may either draw upon or add to its nutrient pools, or the current stockpile of amino acids, carbohydrates, and fats, depending on the needs of the body. These pools are interconvertable, meaning that one nutrient may be converted into another type, because their metabolic pathways are linked by certain intermediate molecules (Figure 16.5.1).
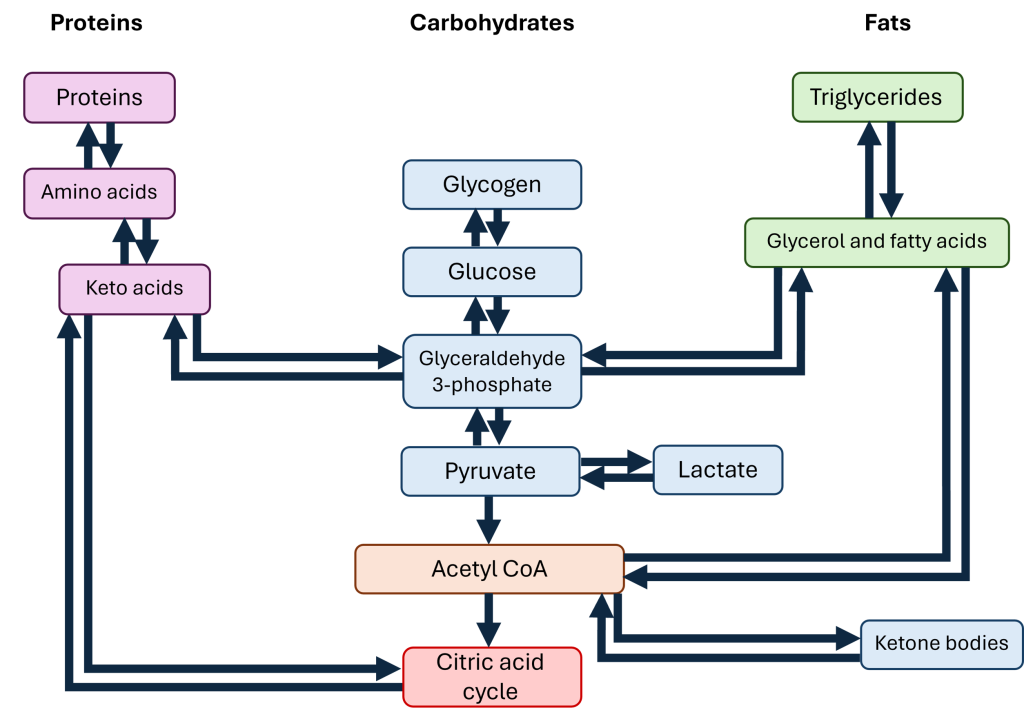
The amino acid pool includes all of the free amino acids within the body. Some of these amino acids will be used to build proteins that will later be lost, such as the keratin produced by cells of the epidermis in the skin; when those skin cells are sloughed off, their proteins are lost with them. The body replaces these lost amino acids through the diet or from breaking down proteins in other tissues; for this reason, the proteins within cells of the body (primarily skeletal muscle) function as a reservoir for amino acids. Free amino acids are available to be incorporated into new proteins within cells or form products derived from amino acids like certain neurotransmitters and hormones. If there are excess amino acids within the body, the liver converts some amino acids to glucose; in this process, the amino group is removed and converted to urea, which is excreted in urine. The rest of the deaminated amino acid can then be converted to glucose, which can then be turned into fat, stored as glycogen, or broken down for ATP.
For carbohydrates, the main storage molecule is glycogen, a polymer of glucose. Some cells, like skeletal muscle, maintain a stockpile of glycogen for the cell’s own use; when skeletal muscle breaks glycogen down into individual glucose molecules, those glucose molecules stay within the skeletal muscle cells to power ATP production. In other tissues, specifically the liver, the glucose released from glycogen breakdown is shuttled to the blood in order to keep blood glucose levels stable. Excess carbohydrates are converted to fats for long-term storage. Since carbohydrates are easily converted to fats, often the carbohydrate pool and the fat pool are considered together.
The fat pool is stored in the form of triglycerides, where one molecule of glycerol (a three-carbon alcohol) is attached to three fatty acids. Triglycerides are primarily stored within adipose tissue. When triglycerides are broken down to glycerol and fatty acids, these two products have different fates. The glycerol may be converted to glucose, but fatty acids can not be converted to glucose. Instead, fatty acids may be converted to acetyl CoA and broken down for ATP via the citric acid cycle; in the liver, acetyl CoA may also be used to produce cholesterol, a key component for cell membranes and the precursor to all steroid hormones. Free fatty acids may also be used by cells and incorporated into cell membranes.
The Absorptive State
The absorptive state, or the fed state, occurs after a meal when your body is digesting the food and absorbing the nutrients (anabolism exceeds catabolism). Digestion begins the moment you put food into your mouth, as the food is broken down into its constituent parts to be absorbed through the intestine. The digestion of carbohydrates begins in the mouth, whereas the digestion of proteins and fats begins in the stomach and small intestine. The constituent parts of these carbohydrates, fats, and proteins are transported across the intestinal wall and enter the bloodstream (sugars and amino acids) or the lymphatic system (fats). From the intestines, these systems transport them to the liver, adipose tissue, or muscle cells that will process and use, or store, the energy.
Depending on the amounts and types of nutrients ingested, the absorptive state can linger for up to 4 hours. The ingestion of food and the rise of glucose concentrations in the bloodstream stimulate pancreatic beta cells to release insulin into the bloodstream, where it initiates the absorption of blood glucose by liver hepatocytes, and by adipose and muscle cells. Once inside these cells, glucose is immediately converted into glucose-6-phosphate. By doing this, a concentration gradient is established where glucose levels are higher in the blood than in the cells. This allows for glucose to continue moving from the blood to the cells where it is needed. Insulin also stimulates glycogenesis, the storage of glucose as glycogen in the liver and muscle cells where it can be used for later energy needs of the body. Insulin also promotes the synthesis of protein in muscle. As you will see, muscle protein can be catabolized and used as fuel in times of starvation.
If energy is exerted shortly after eating, the dietary fats and sugars that were just ingested will be processed and used immediately for energy. If not, the excess glucose is stored as glycogen in the liver and muscle cells, or as fat in adipose tissue; excess dietary fat is also stored as triglycerides in adipose tissues.
Figure 16.5.2 summarizes the metabolic processes occurring in the body during the absorptive state.

The Postabsorptive State
The postabsorptive state, or the fasting state, occurs when the food has been digested, absorbed, and stored. You commonly fast overnight, but skipping meals during the day puts your body in the postabsorptive state as well. During this state, the body must rely initially on stored glycogen. Glucose levels in the blood begin to drop as it is absorbed and used by the cells. In response to the decrease in glucose, insulin levels also drop. Glycogen and triglyceride storage slows. However, due to the demands of the tissues and organs, blood glucose levels must be maintained in the normal range of 80 to 120 mg/dL. In response to a drop in blood glucose concentration, the hormone glucagon is released from the alpha cells of the pancreas. Glucagon acts upon the liver cells, where it inhibits the synthesis of glycogen and stimulates glycogenolysis, the breakdown of stored glycogen back into glucose. This glucose is released from the liver to be used by the peripheral tissues and the brain. As a result, blood glucose levels begin to rise. Gluconeogenesis will also begin in the liver to replace the glucose that has been used by the peripheral tissues.
After ingestion of food, fats and proteins are processed as described previously; however, the glucose processing changes a bit. The peripheral tissues preferentially absorb glucose. The liver, which normally absorbs and processes glucose, will not do so after a prolonged fast. The gluconeogenesis that has been ongoing in the liver will continue after fasting to replace the glycogen stores that were depleted in the liver. After these stores have been replenished, excess glucose that is absorbed by the liver will be converted into triglycerides and fatty acids for long-term storage. Figure 16.5.3 summarizes the metabolic processes occurring in the body during the postabsorptive state.

Blood Glucose Homeostasis
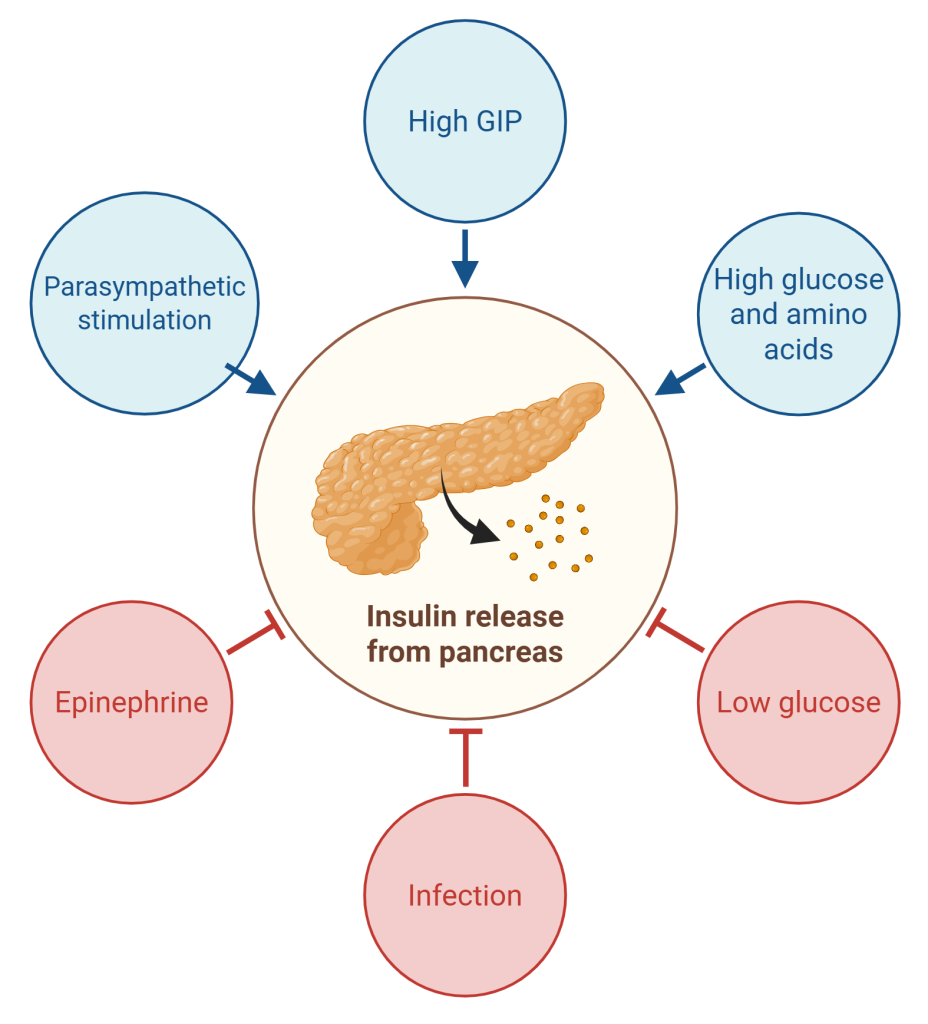
Blood glucose levels in the body are controlled by two major hormones: insulin and glucagon. These hormones are antagonistic, meaning that their effects are opposite one another. During the absorptive state, insulin is released due to several factors (Figure 16.5.4):
- Rising blood glucose levels and free amino acid levels stimulate the pancreas to release insulin.
- Enteroendocrine cells of the small intestine secrete glucose-dependent insulinotropic peptide (GIP) into the blood, which interact with receptors on beta cells to stimulate the release of insulin.
- The parasympathetic division of the autonomic nervous system stimulates insulin release during feeding.
Insulin binds to receptors on skeletal muscle, adipose, and liver cells, which promotes the insertion of glucose carrier proteins into the plasma membrane (Figure 16.5.5). With more glucose transport proteins embedded in the membrane, glucose is rapidly moved into the target cells for breakdown, glycogen synthesis, or conversion to fats for long-term storage.
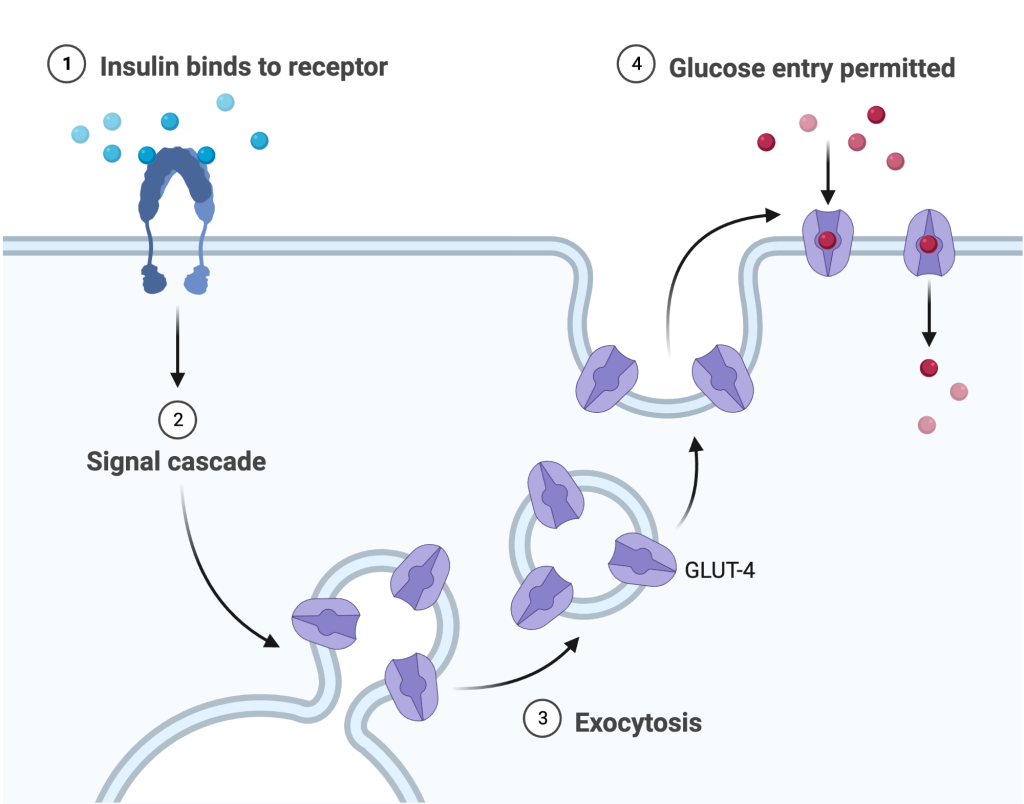
As glucose is rapidly taken up by target cells, the pancreas detects the falling glucose levels and reduces insulin secretion in a classic example of negative feedback regulation.
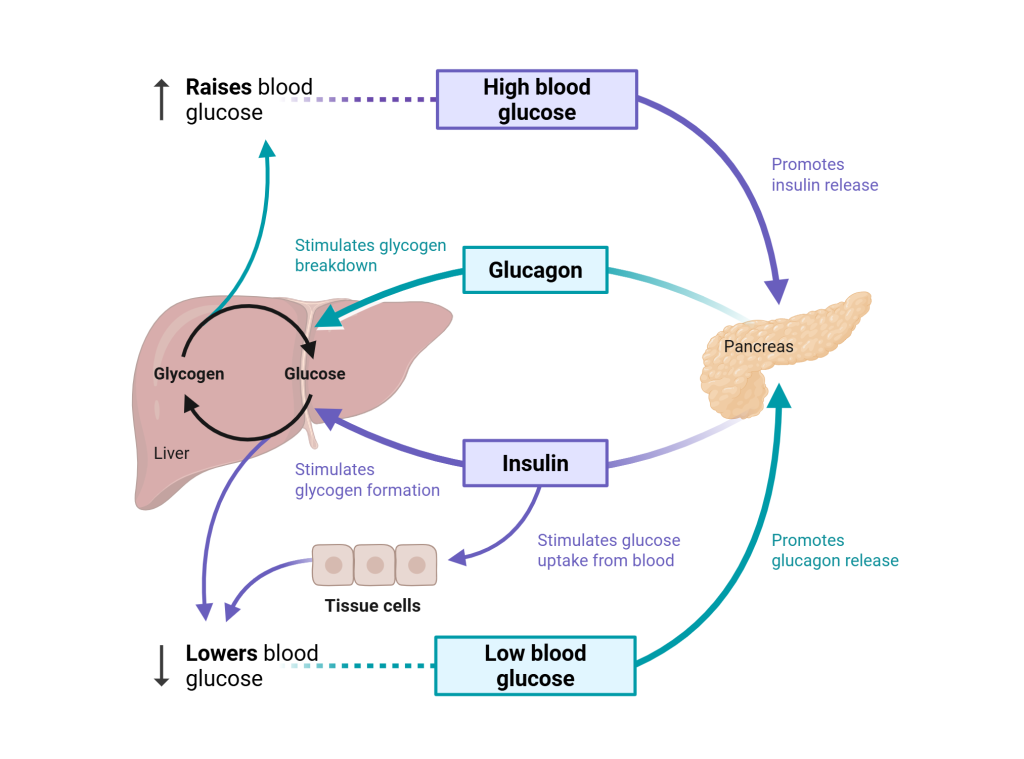
Glucagon is an antagonist of insulin, so its pathway is in many ways the opposite of insulin. When blood glucose levels fall below normal in the postabsorptive state, glucagon is released from alpha cells in the pancreas. Glucagon directs the liver and skeletal muscle to break down the stored glycogen (glycogenolysis), adipose tissue and the liver to break down lipids (lipolysis), and the liver to produce glucose from amino acids or glycerol (gluconeogenesis). Combined, these actions help to raise blood glucose levels back to the normal range. Once blood glucose levels rise to normal, glucagon secretion decreases.
As shown in Figure 16.5.6, glucagon and insulin work together to maintain blood glucose levels within the normal homeostatic range of 80 to 120 mg/dL. Maintaining blood glucose levels within this range is important, as the brain almost exclusively uses glucose as its energy source.
Clinical Connection – Diabetes
Diabetes is a chronic condition where blood glucose levels are too high. This could be due to an autoimmune disorder where the beta cells of the pancreas which produce insulin have been destroyed; this results in type 1 diabetes. In type 2 diabetes, the pancreas is able to produce insulin but the target cells are not responding to keep blood glucose within a normal range; in this case, the target cells have become resistant to insulin and do not respond appropriately. This is the most common type of diabetes. According to the CDC, a total of 38.4 million people in the United States had diabetes in 2021, accounting for 11.6% of the US population.
While diabetes can impact anyone, there are some communities with higher frequency of diabetes diagnosis and inequity in access to care:
- Socioeconomic Status: Those who earn less than $80,000 a year have three times the risk of developing diabetes than those who earn more. Education level also showed differences in prevalence, with 13.1% of adults with less than a high school education having a diabetes diagnosis, compared to 6.9% of those with more than a high school education.
- Race and Ethnic Background: Between 2019 and 2021, diagnosis of diabetes was highest among American Indian and Alaska Native (AI/AN) adults, where 13.6% of the AI/AN population was diagnosed with diabetes. The next highest was within non-Hispanic Black adults, with a diabetes prevalence of 12.1%, followed by adults of Hispanic origin at 11.7%.
- Residence: Prevalence of diabetes is higher among adults living in rural areas (9.5% of residents) compared to metropolitan areas (8.1% of residents).
Each community is different, with unique challenges in education, food stability, housing stability, poverty, and access to medical care. Reducing the health disparities in these communities will require working with community leaders to find solutions together. A 5-year project funded by the Merck Foundation looked into ways to improve diabetes care and reduce health disparities in patients with diabetes. Watch “Key Takeaways from Bridging the Gap: Reducing Disparities in Diabetes Care” by BTG: Reducing Disparities in Diabetes Care (May 1, 2023) below or follow this link to hear the main takeaways from this project and their practical suggestions for improving patient care.
Starvation
When the body is deprived of nourishment for an extended period of time, it goes into “survival mode.” The first priority for survival is to provide enough glucose or fuel for the brain. The second priority is the conservation of amino acids for proteins. Therefore, the body uses ketones to satisfy the energy needs of the brain and other glucose-dependent organs, and to maintain proteins in the cells. Because glucose levels are very low during starvation, glycolysis will shut off in cells that can use alternative fuels. For example, muscles will switch from using glucose to fatty acids as fuel. As previously explained, fatty acids can be converted into acetyl CoA and processed through the Krebs cycle to make ATP. Pyruvate, lactate, and alanine from muscle cells are not converted into acetyl CoA and used in the Krebs cycle, but are exported to the liver to be used in the synthesis of glucose. As starvation continues, and more glucose is needed, glycerol from fatty acids can be liberated and used as a source for gluconeogenesis.
After several days of starvation, ketone bodies become the major source of fuel for the heart and other organs. As starvation continues, fatty acids and triglyceride stores are used to create ketones for the body. This prevents the continued breakdown of proteins that serve as carbon sources for gluconeogenesis. Once these stores are fully depleted, proteins from muscles are released and broken down for glucose synthesis. Overall survival is dependent on the amount of fat and protein stored in the body.
Section Review
To meet the body’s constant energy demands—especially for the brain—nutrients from food are both used immediately and stored for later through interconnected metabolic pathways involving carbohydrates, fats, and proteins. During the absorptive (fed) state, insulin facilitates glucose uptake and promotes the storage of energy as glycogen and fat, while nutrients are absorbed into tissues for immediate use or storage. In contrast, during the postabsorptive (fasting) state, glucagon stimulates the breakdown of glycogen, lipids, and proteins to maintain blood glucose levels, primarily for brain function. Blood glucose homeostasis is regulated by the opposing actions of insulin and glucagon through negative feedback mechanisms. In starvation, the body shifts to using fatty acids and ketones to conserve protein and protect essential functions. Disruption in these regulatory systems, such as in diabetes, results in chronic elevated blood glucose and reflects broader socioeconomic and healthcare disparities that affect disease prevalence and outcomes.
Review Questions
Critical Thinking Questions
Glossary
- absorptive state
- also called the fed state; the metabolic state occurring during the first few hours after ingesting food in which the body is digesting food and absorbing the nutrients
- glucagon
- hormone secreted by the pancreas that stimulates the release of glucose from storage sites
- glycogen
- form that glucose assumes when it is stored
- glycogenesis
- the formation of glycogen from glucose
- insulin
- hormone secreted by the pancreas that stimulates the uptake of glucose into the cells
- lipolysis
- the breakdown of fats and other lipids to release fatty acids
- postabsorptive state
- also called the fasting state; the metabolic state occurring after digestion when food is no longer the body’s source of energy and it must rely on stored glycogen
Glossary Flashcards
References
Peek, M. E., Cargill, A., & Huang, E. S. (2007, October). Diabetes health disparities: a systematic review of health care interventions. Medical care research and review: MCRR 64(5 Suppl), 101S–56S.
Hassan, S., Gujral, U. P., Quarells, R. C., Rhodes, E. C., Shah, M. K., Obi, J., Lee, W. H., Shamambo, L., Weber, M. B., & Narayan, K. M. V. (2023, June 22). Disparities in diabetes prevalence and management by race and ethnicity in the USA: defining a path forward. Lancet. Diabetes & endocrinology 11(7), 509–524.
Centers for Disease Control and Prevention. (2024, May 15). Advancing health equity: Diabetes.
American Diabetes Association. (n.d.). Committment to health care access.
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
