Chapter 18. The Sexual Systems
18.4 Physiology of the Male Sexual System
Learning Objectives
By the end of this section, you will be able to:
- diagram or describe the location of spermatogenesis in the seminiferous tubule, including the locations of and functions of nurse (sustentacular [Sertoli]) and interstitial [Leydig] cells;
- describe the pathway of sperm from the seminiferous tubules to the external urethral orifice of the penis; and
- diagram or describe the hypothalamic-pituitary-gonad axis and the feedback loops that control the production and regulation of androgens, inhibin, and androgen-binding protein in the testis.
The least mature cells, the spermatogonia (singular spermatogonium), line the basement membrane inside the tubule. Spermatogonia are the stem cells of the testis, which means that they are still able to differentiate into a variety of different cell types throughout adulthood. Spermatogonia divide to produce primary and secondary spermatocytes, then spermatids, which finally produce formed sperm. The process that begins with spermatogonia and concludes with the production of sperm is called spermatogenesis.
Spermatogenesis
Spermatogenesis occurs in the seminiferous tubules that form the bulk of each testis. The process begins at puberty, after which time sperm are produced constantly throughout a man’s life. One production cycle, from spermatogonia through formed sperm, takes approximately 64 days. A new cycle starts approximately every 16 days, although this timing is not synchronous across the seminiferous tubules. Sperm counts—the total number of sperm a man produces—slowly decline after age 35, and some studies suggest that smoking can lower sperm counts irrespective of age.
The process of spermatogenesis begins with mitosis of the diploid spermatogonia (Figure 18.4.1). Because these cells are diploid (2n), they each have a complete copy of the father’s genetic material, or 46 chromosomes. However, mature gametes are haploid (1n), containing 23 chromosomes—meaning that daughter cells of spermatogonia must undergo a second cellular division through the process of meiosis.
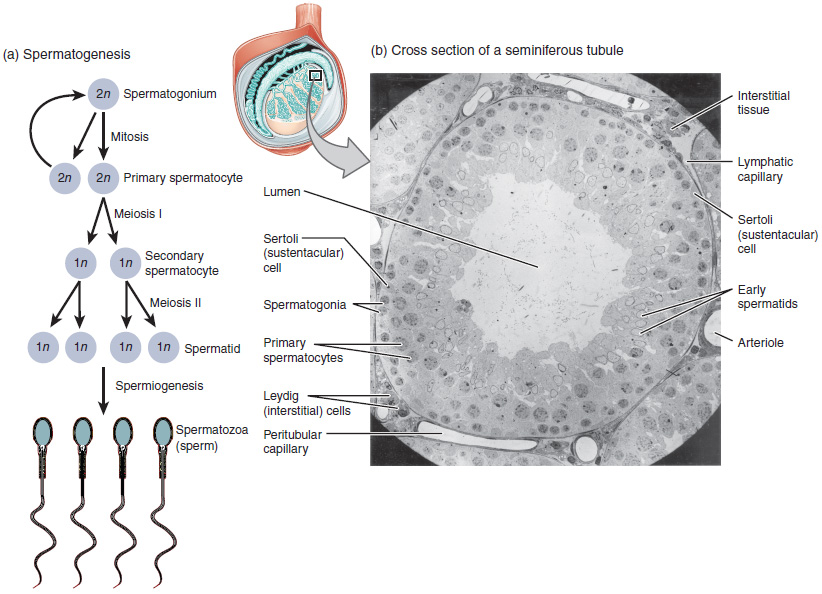
Two identical diploid cells result from spermatogonia mitosis. One of these cells remains a spermatogonium, and the other becomes a primary spermatocyte, the next stage in the process of spermatogenesis. As in mitosis, DNA is replicated in a primary spermatocyte, before it undergoes a cell division called meiosis I. During meiosis I each of the 23 pairs of chromosomes separates. This results in two cells, called secondary spermatocytes, each with only half the number of chromosomes. Now a second round of cell division (meiosis II) occurs in both of the secondary spermatocytes. During meiosis II each of the 23 replicated chromosomes divides, similar to what happens during mitosis. Thus, meiosis results in separating the chromosome pairs. This second meiotic division results in a total of four cells with only half of the number of chromosomes. Each of these new cells is a spermatid. Although haploid, early spermatids look very similar to cells in the earlier stages of spermatogenesis, with a round shape, central nucleus, and large amount of cytoplasm. A process called spermiogenesis transforms these early spermatids, reducing the cytoplasm, and beginning the formation of the parts of a true sperm. The fifth stage of germ cell formation—spermatozoa, or formed sperm—is the end result of this process, which occurs in the portion of the tubule nearest the lumen. Eventually, the sperm are released into the lumen and are moved along a series of ducts in the testis toward a structure called the epididymis for the next step of sperm maturation.
Structure of Formed Sperm
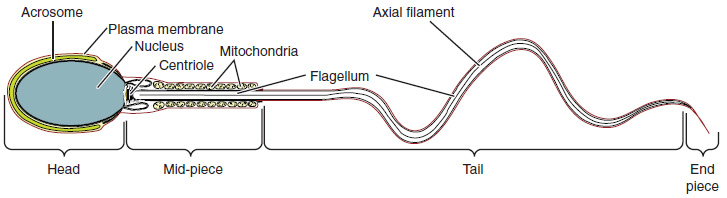
Sperm are smaller than most cells in the body; in fact, the volume of a sperm cell is 85,000 times less than that of the female gamete. Approximately 100 to 300 million sperm are produced each day, whereas women typically ovulate only one oocyte per month. As is true for most cells in the body, the structure of sperm cells speaks to their function. Sperm have a distinctive head, mid-piece, and tail region (Figure 18.4.2). The head of the sperm contains the extremely compact haploid nucleus with very little cytoplasm. These qualities contribute to the overall small size of the sperm (the head is only 5 μm long). A structure called the acrosome covers most of the head of the sperm cell as a “cap” that is filled with lysosomal enzymes important for preparing sperm to participate in fertilization. Tightly packed mitochondria fill the mid-piece of the sperm. ATP produced by these mitochondria will power the flagellum, which extends from the neck and the mid-piece through the tail of the sperm, enabling it to move the entire sperm cell. The central strand of the flagellum, the axial filament, is formed from one centriole inside the maturing sperm cell during the final stages of spermatogenesis.
Sperm Transport
To fertilize an egg, sperm must be moved from the seminiferous tubules in the testes, through the epididymis, and—later during ejaculation—along the length of the penis and out into the female reproductive tract.
Testosterone
Testosterone, an androgen, is a steroid hormone produced by Leydig cells. The alternate term for Leydig cells, interstitial cells, reflects their location between the seminiferous tubules in the testes. In male embryos, testosterone is secreted by Leydig cells by the seventh week of development, with peak concentrations reached in the second trimester. This early release of testosterone results in the anatomical differentiation of the male sexual organs. In childhood, testosterone concentrations are low. They increase during puberty, activating characteristic physical changes and initiating spermatogenesis.
Functions of Testosterone
The continued presence of testosterone is necessary to keep the male reproductive system working properly, and Leydig cells produce approximately 6 to 7 mg of testosterone per day. Testicular steroidogenesis (the manufacture of androgens, including testosterone) results in testosterone concentrations that are 100 times higher in the testes than in the circulation. Maintaining these normal concentrations of testosterone promotes spermatogenesis, whereas low levels of testosterone can lead to infertility. In addition to intratesticular secretion, testosterone is also released into the systemic circulation and plays an important role in muscle development, bone growth, the development of secondary sex characteristics, and maintaining libido (sex drive) in both males and females. In females, the ovaries secrete small amounts of testosterone, although most is converted to estradiol. A small amount of testosterone is also secreted by the adrenal glands in both sexes.
Control of Testosterone
The regulation of testosterone concentrations throughout the body is critical for male reproductive function. The intricate interplay between the endocrine system and the reproductive system is shown in Figure 18.4.3.
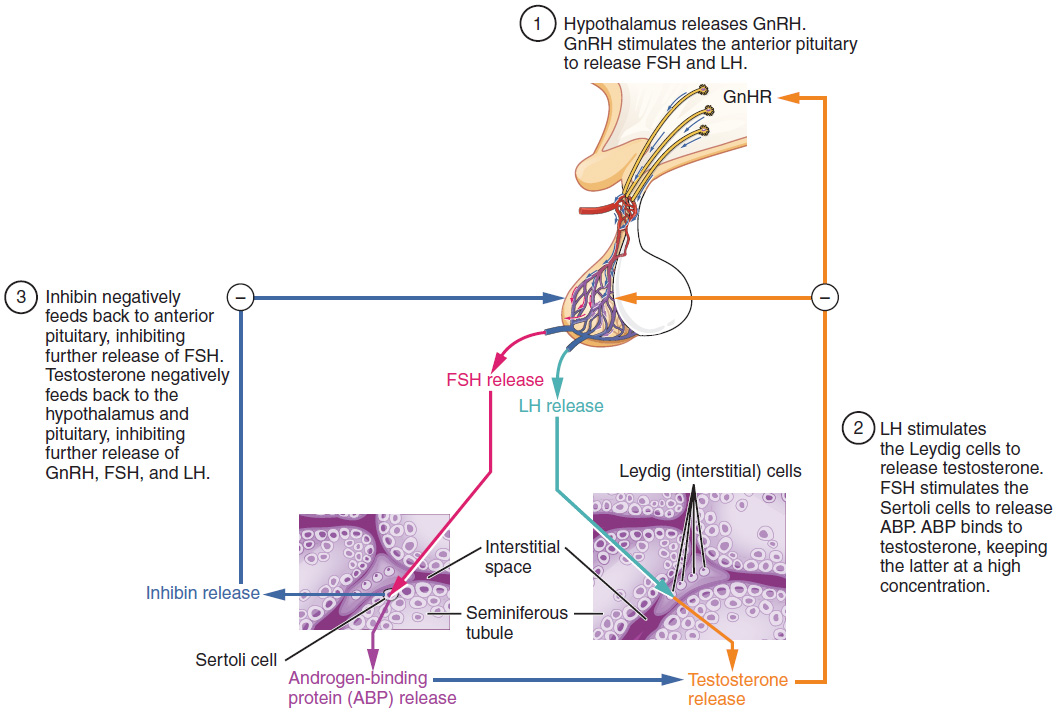
The regulation of Leydig cell production of testosterone begins outside of the testes. The hypothalamus and the pituitary gland in the brain integrate external and internal signals to control testosterone synthesis and secretion. The regulation begins in the hypothalamus. Pulsatile release of a hormone called gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the endocrine release of hormones from the pituitary gland. Binding of GnRH to its receptors on the anterior pituitary gland stimulates release of the two gonadotropins: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These two hormones are critical for reproductive function in both men and women. In men, FSH binds predominantly to the Sertoli cells within the seminiferous tubules to promote spermatogenesis. FSH also stimulates the Sertoli cells to produce hormones called inhibins, which function to inhibit FSH release from the pituitary, thus reducing testosterone secretion. These polypeptide hormones correlate directly with Sertoli cell function and sperm number; inhibin B can be used as a marker of spermatogenic activity. In men, LH binds to receptors on Leydig cells in the testes and upregulates the production of testosterone.
A negative feedback loop predominantly controls the synthesis and secretion of both FSH and LH. Low blood concentrations of testosterone stimulate the hypothalamic release of GnRH. GnRH then stimulates the anterior pituitary to secrete LH into the bloodstream. In the testis, LH binds to LH receptors on Leydig cells and stimulates the release of testosterone. When concentrations of testosterone in the blood reach a critical threshold, testosterone itself will bind to androgen receptors on both the hypothalamus and the anterior pituitary, inhibiting the synthesis and secretion of GnRH and LH, respectively. When the blood concentrations of testosterone once again decline, testosterone no longer interacts with the receptors to the same degree and GnRH and LH are once again secreted, stimulating more testosterone production. This same process occurs with FSH and inhibin to control spermatogenesis.
Aging and the Male Reproductive System
Declines in Leydig cell activity can occur in males beginning at 40 to 50 years of age. The resulting reduction in circulating testosterone concentrations can lead to symptoms of andropause, also known as male menopause. While the reduction in sex steroids is akin to female menopause, there is no clear sign—such as a lack of a menstrual period—to denote the initiation of andropause. Instead, people with this condition report feelings of fatigue, reduced muscle mass, depression, anxiety, irritability, loss of libido, and insomnia. A reduction in spermatogenesis resulting in lowered fertility is also reported, and sexual dysfunction can also be associated with andropausal symptoms.
Whereas some researchers believe that certain aspects of andropause are difficult to tease apart from aging in general, testosterone replacement is sometimes prescribed to alleviate some symptoms. Recent studies have shown a benefit from androgen replacement therapy on the new onset of depression in elderly males; however, other studies caution against testosterone replacement for long-term treatment of andropause symptoms, showing that high doses can sharply increase the risk of both heart disease and prostate cancer.
Section Review
Spermatogenesis begins with mitotic division of spermatogonia (stem cells) to produce primary spermatocytes that undergo the two divisions of meiosis to become secondary spermatocytes, then the haploid spermatids. During spermiogenesis, spermatids are transformed into spermatozoa (formed sperm). Upon release from the seminiferous tubules, sperm are moved to the epididymis where they continue to mature. During ejaculation, sperm exit the epididymis through the ductus deferens, a duct in the spermatic cord that leaves the scrotum. The ampulla of the ductus deferens meets the seminal vesicle, a gland that contributes fructose and proteins, at the ejaculatory duct. The fluid continues through the prostatic urethra, where secretions from the prostate are added to form semen. These secretions help the sperm to travel through the urethra and into the female reproductive tract. Secretions from the bulbourethral glands protect sperm and cleanse and lubricate the penile (spongy) urethra.
Testosterone is the main male sex hormone and is vital for normal function of the male reproductive system. Similar to the female reproductive system, testosterone levels are regulated by GnRH, LSH, and FSH. Testosterone exerts negative feedback on LH and FSH. In addition, inhibin functions to inhibit FSH release.
Review Questions
Critical Thinking Questions
Glossary
-
- Leydig cells
- cells between the seminiferous tubules of the testes that produce testosterone; a type of interstitial cell
- sperm
- male gamete (also spermatozoon)
- spermatid
- immature sperm cells produced by meiosis II of secondary spermatocytes
- spermatocyte
- cell that results from the division of spermatogonium and undergoes meiosis I and meiosis II to form spermatids
- spermatogenesis
- formation of new sperm, occurs in the seminiferous tubules of the testes
- spermatogonia
- diploid precursor cells that become sperm (singular spermatogonium)
- spermiogenesis
- transformation of spermatids to spermatozoa during spermatogenesis
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
