Chapter 4. Membranes and Transport
4.1 Body Fluids and Fluid Compartments
Learning Objectives
By the end of this section, you will be able to:
- Explain the importance of water in the body
- Contrast the composition of the intracellular fluid with that of the extracellular fluid
- Explain the importance of protein channels in the movement of solutes
The chemical reactions of life take place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human body, solutes vary in different parts of the body but may include proteins—including those that transport lipids, carbohydrates, and, very importantly, electrolytes. Often in medicine, an electrolyte is referred to as a mineral dissociated from a salt that carries an electrical charge (an ion). For instance, sodium ions (Na+) and chloride ions (Cl–) are often referred to as electrolytes.
In the body, water moves through semipermeable membranes of cells and from one compartment of the body to another by a process called osmosis, which will be discussed later in this chapter. As a result, water will move into and out of cells and tissues, depending on the relative concentrations of the water and solutes found there. An appropriate balance of solutes inside and outside of cells must be maintained to ensure normal function.
Body Water Content
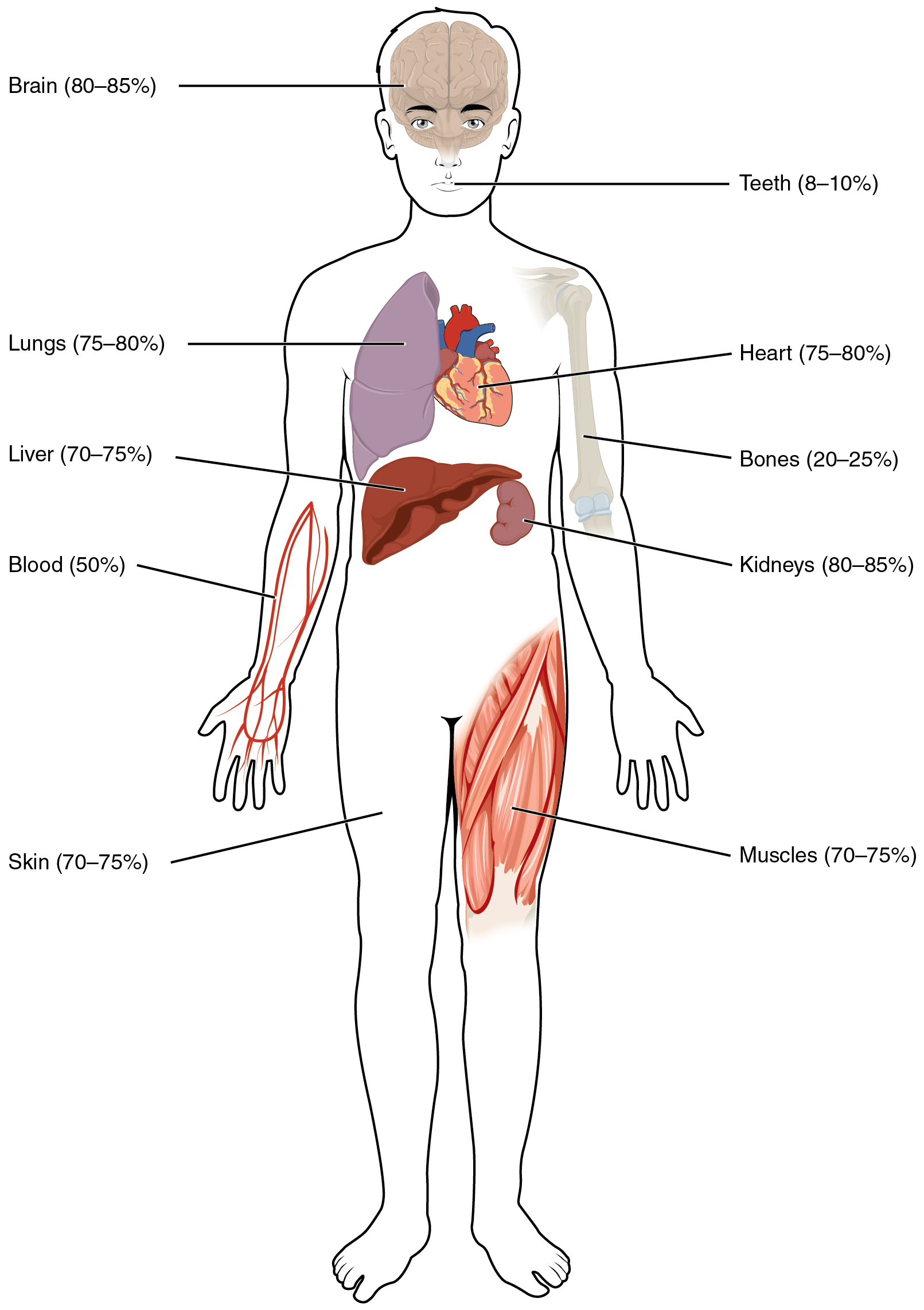
Human beings are mostly water, ranging from about 75% of body mass in infants to about 50% to 60% in adult men and women to as low as 45% in old age. The percent of body water changes with development, because the proportions of the body given over to each organ and to muscles, fat, bone, and other tissues change from infancy to adulthood (Figure 4.1.1). Your brain and kidneys have the highest proportions of water, which composes 80% to 85% of their masses. In contrast, teeth have the lowest proportion of water, at 8% to 10%.
Fluid Compartments
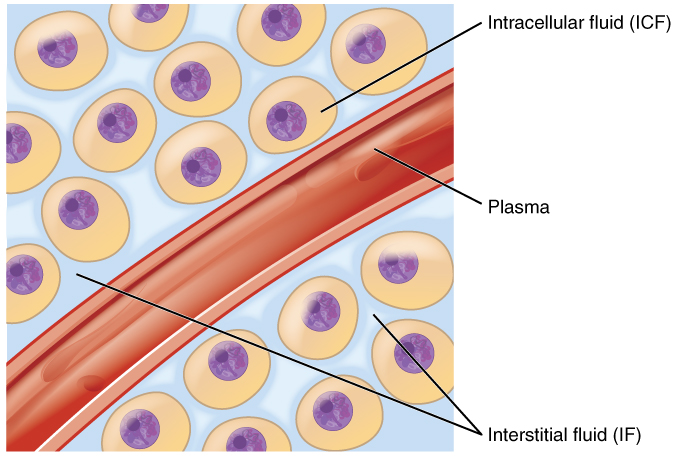
Body fluids can be discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some form of a physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the body. Extracellular fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells not in the blood (Figure 4.1.2).
Intracellular Fluid
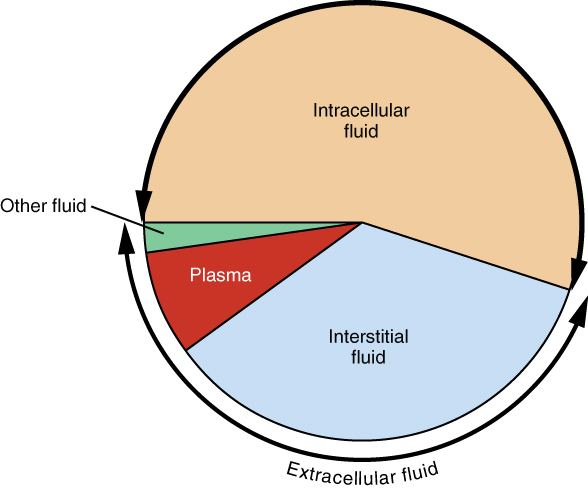
The ICF lies within cells and is the principal component of the cytosol/cytoplasm. The ICF makes up about 60% of the total water in the human body, and in an average-size adult male, the ICF accounts for about 25 liters (seven gallons) of fluid (Figure 4.1.3). This fluid volume tends to be very stable, because the amount of water in living cells is closely regulated. If the amount of water inside a cell falls to a value that is too low, the cytosol becomes too concentrated with solutes to carry on normal cellular activities; if too much water enters a cell, the cell may burst and be destroyed.
Extracellular Fluid
The ECF accounts for the other one-third of the body’s water content. Approximately 20% of the ECF is found in plasma. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Gases, nutrients, and waste materials travel between capillaries and cells through the IF. Cells are separated from the IF by a selectively permeable cell membrane that helps regulate the passage of materials between the IF and the interior of the cell.
The body has other water-based ECF. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humor of the eye. Because these fluids are outside of cells, these fluids are also considered components of the ECF compartment.
Composition of Body Fluids
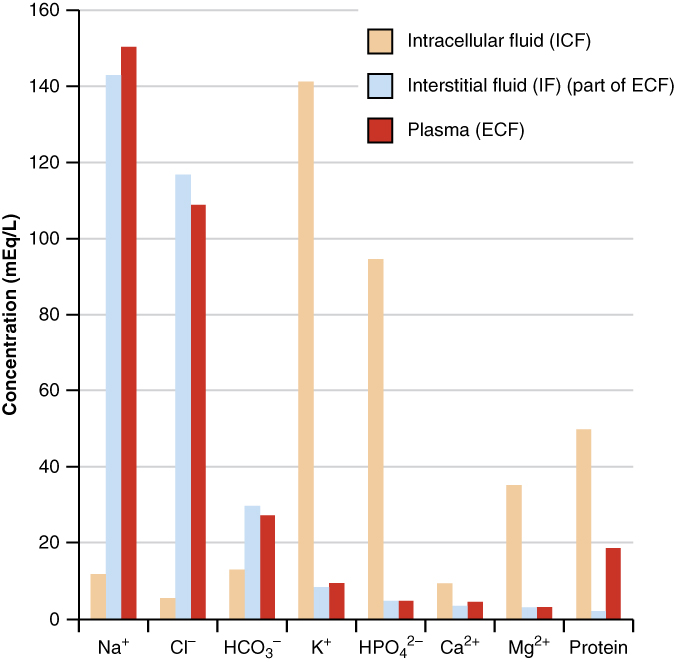
The compositions of the two components of the ECF—plasma and IF—are more similar to each other than either is to the ICF (Figure 4.1.4). Blood plasma has high concentrations of sodium, chloride, bicarbonate, and protein. The IF has high concentrations of sodium, chloride, and bicarbonate but a relatively lower concentration of protein. In contrast, the ICF has elevated amounts of potassium, phosphate, magnesium, and protein. Overall, the ICF contains high concentrations of potassium and phosphate (HPO42−), whereas both plasma and the ECF contain high concentrations of sodium and chloride.
External Website

Watch this video to learn more about body fluids, fluid compartments, and electrolytes. When blood volume decreases due to sweating, from what source is water taken in by the blood?
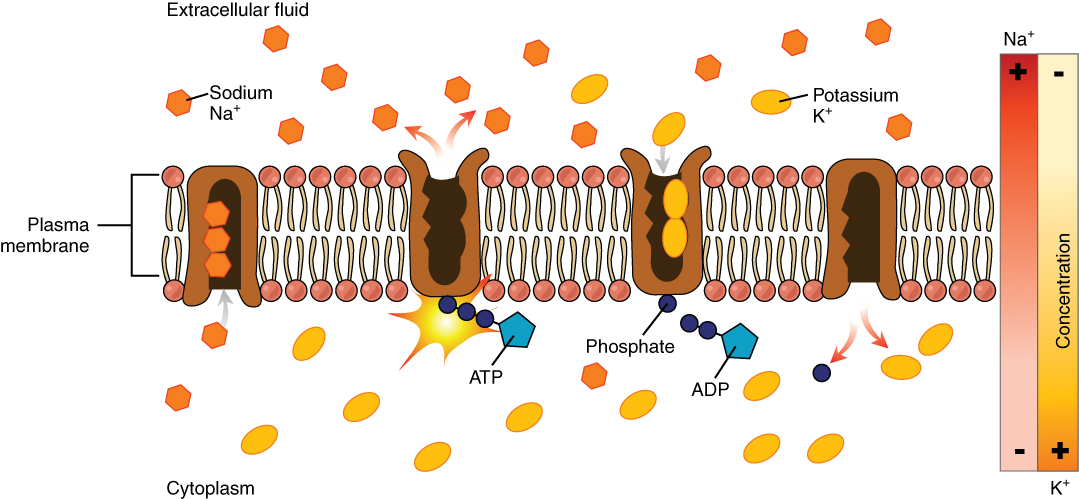
Most body fluids are neutral in charge. Thus, cations (positively charged ions) and anions (negatively charged ions) are balanced in fluids. As seen in the previous graph, sodium (Na+) ions and chloride (Cl–) ions are concentrated in the ECF of the body, whereas potassium (K+) ions are concentrated inside cells. Although sodium and potassium can “leak” through “pores” into and out of cells, respectively, the high levels of potassium and low levels of sodium in the ICF are maintained by sodium-potassium pumps in the cell membranes. These pumps use the energy supplied by ATP to pump sodium out of the cell and potassium into the cell (Figure 4.1.5).
Section Review
Your body is mostly water. Body fluids are aqueous solutions with differing concentrations of materials, called solutes. An appropriate balance of water and solute concentrations must be maintained to ensure cellular functions. If the cytosol becomes too concentrated due to water loss, cell functions deteriorate. If the cytosol becomes too dilute due to water intake by cells, cell membranes can be damaged, and the cell can burst. Fluid is present in three major fluid compartments: intracellular fluid within cells and extracellular fluid, which includes interstitial fluid between cells in a tissue and plasma, the fluid component of blood. Fluid can move between these compartments depending on solute concentration and the needs of a cell.
Interactive Link Questions
Watch this video to learn more about body fluids, fluid compartments, and electrolytes.
Video 4.1. Fluids and Electrolytes Part 1 by Dr. John Campbell
Review Questions
Critical Thinking Questions
Glossary
- extracellular fluid (ECF)
- fluid exterior to cells; includes the interstitial fluid, blood plasma, and fluids found in other reservoirs in the body
- fluid compartment
- a location containing fluid that is separated from other locations by a physical barrier
- interstitial fluid (IF)
- fluid in the small spaces between cells not contained within blood vessels
- intracellular fluid (ICF)
- fluid in the cytosol of cells
- plasma
- the fluid component of blood
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Image Descriptions
Figure 4.1.1. This is an anatomical illustration displaying the water content of various organs and tissues in the human body. The diagram uses a front-facing human figure with labeled organs and body parts. Text labels with percentages include: “Brain (80–85%)”, “Teeth (8–10%)”, “Lungs (75–80%)”, “Heart (75–80%)”, “Liver (70–75%)”, “Bones (20–25%)”, “Blood (50%)”, “Kidneys (80–85%)”, “Skin (70–75%)”, and “Muscles (70–75%)”. The illustration uses different colors to distinguish between organs and systems, with the brain and kidneys having the highest water content and teeth having the lowest. [Return to Figure 4.1.1]
Figure 4.1.2. This is a medical illustration showing the distribution of body fluids at the cellular level. The diagram depicts round cells with purple nuclei surrounded by a light blue background. A red blood vessel runs diagonally through the image. Text labels identify three fluid compartments: “Intracellular fluid (ICF)” pointing to the fluid inside the cells, “Interstitial fluid (IF)” pointing to the fluid in the spaces between cells, and “Plasma” pointing to the fluid within the blood vessel. The cells are shown as orange/peach-colored circles with darker purple nuclei in their centers. [Return to Figure 4.1.2]
Figure 4.1.3. This is a pie chart illustrating the distribution of body fluids across different compartments. The circular diagram is divided into segments of varying sizes with text labels. The largest segment (tan/beige) is labeled “Intracellular fluid” occupying roughly the top half of the circle. The lower portion shows “Interstitial fluid” (light blue) as the second largest segment. A smaller red triangular segment is labeled “Plasma,” and a tiny green segment is labeled “Other fluid.” A curved bracket along the bottom arc of the circle indicates “Extracellular fluid,” which encompasses the interstitial fluid, plasma, and other fluid compartments combined. [Return to Figure 4.1.3]
Figure 4.1.4. This is a scientific bar graph showing the distribution of electrolytes and proteins across three body fluid compartments. The y-axis shows “Concentration (mEq/L)” ranging from 0 to 160, and the x-axis lists eight substances with their chemical symbols: “Na⁺”, “Cl⁻”, “HCO₃⁻”, “K⁺”, “HPO₄²⁻”, “Ca²⁺”, “Mg²⁺”, and “Protein”. The legend indicates three categories represented by different colored bars: “Intracellular fluid (ICF)” (tan/beige), “Interstitial fluid (IF) (part of ECF)” (light blue), and “Plasma (ECF)” (red/dark red). The graph demonstrates that sodium and chloride concentrations are highest in extracellular fluids (plasma and interstitial fluid), while potassium and protein concentrations are highest in intracellular fluid. Each substance has up to three bars showing its concentration in the different fluid compartments. [Return to Figure 4.1.4]
Figure 4.1.5. This illustration depicts active transport across a cell plasma membrane via the sodium-potassium pump. The diagram shows a cross-section with a phospholipid bilayer (pink circles as heads, white lines as tails) containing brown protein channels. Text labels identify “Extracellular fluid” (top), “Cytoplasm” (bottom), “Plasma membrane” (left), “Sodium Na⁺” (orange hexagons), “Potassium K⁺” (yellow ovals), “ATP”, “ADP”, and “Phosphate”. The pump cycle shows: three sodium ions enter from the cytosol, ATP binds causing the pump to change shape and open to the extracellular fluid, sodium is released and two potassium ions enter, ADP detaches leaving phosphate attached, the pump changes shape again to release potassium into the cytosol, and phosphate detaches to restart the cycle. A concentration gradient diagram on the right labeled “Concentration” with “Na⁺” (plus and minus signs) and “K⁺” (minus and plus signs) indicates that the pump works against natural concentration gradients, as sodium normally diffuses into the cell while potassium diffuses out. [Return to Figure 4.1.5]
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
