Chapter 4. Membranes and Transport
4.3 Passive Transport
Learning Objectives
By the end of this section, you will be able to:
- Describe how molecules cross the cell membrane based on their properties and concentration gradients
- Use the properties of a molecule or ion to predict if the substance can cross a cell membrane by simple diffusion or if a membrane transport protein or vesicle is required
- Describe the effects of hypertonic, isotonic, and hypotonic solutions on a cell’s volume at equilibrium
- Describe the forces that move water across biological membranes and the role of aquaporins in transmembrane water transport
In order to understand how substances move passively across a cell membrane, it is necessary to understand concentration gradients and diffusion. A concentration gradient is the difference in concentration of a substance across a space. Molecules (or ions) will spread/diffuse from where they are more concentrated to where they are less concentrated until they are equally distributed in that space. (When molecules move in this way, they are said to move down their concentration gradient, from high concentration to low concentration.) Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. A couple of common examples will help to illustrate this concept. Imagine being inside a closed room. If a bottle of perfume were sprayed, the scent molecules would naturally diffuse from the spot where they left the bottle to all corners of the room, and this diffusion would go on until the molecules were equally distributed in the room. Another example is a spoonful of sugar placed in a cup of tea. Eventually the sugar will diffuse throughout the tea until no concentration gradient remains. In both cases, if the room is warmer or the tea hotter, diffusion occurs even faster as the molecules are bumping into each other and spreading out faster than at cooler temperatures.
External Website
 Visit Wikimedia’s webpage to see diffusion, and how it is propelled by the kinetic energy of molecules in solution. How does temperature affect diffusion rate, and why?
Visit Wikimedia’s webpage to see diffusion, and how it is propelled by the kinetic energy of molecules in solution. How does temperature affect diffusion rate, and why?
Whenever a substance exists in greater concentration on one side of a semipermeable membrane, such as cell membranes, any substance that can move down its concentration gradient across the membrane will do so. If the substances can move across the cell membrane without the cell expending energy, the movement of molecules is called passive transport. Consider substances that can easily diffuse through the lipid bilayer of the cell membrane, such as the gases oxygen (O2) and carbon dioxide (CO2). These small fat-soluble gasses and other small lipid-soluble molecules can dissolve in the membrane and enter or exit the cell following their concentration gradient. This mechanism of molecules moving across a cell membrane from the side where they are more concentrated to the side where they are less concentrated is a form of passive transport called simple diffusion. O2 generally diffuses into cells because it is more concentrated outside of them, and CO2 typically diffuses out of cells because it is more concentrated inside of them.
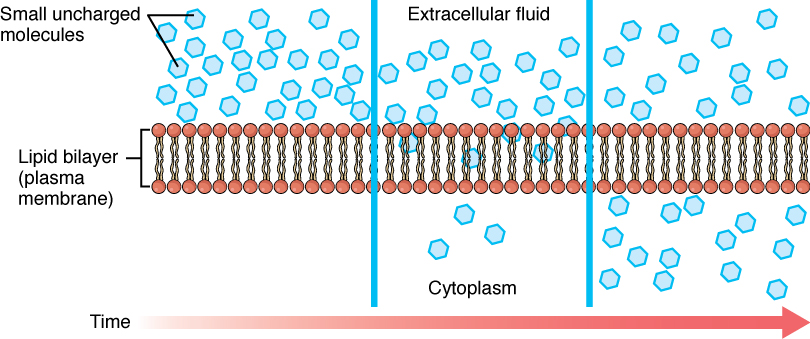
Before moving on, it is important to realize that the concentration gradients for oxygen and carbon dioxide will always exist across a living cell and never reach equal distribution. This is because cells rapidly use up oxygen during metabolism, so there is typically a lower concentration of O2 inside the cell than outside. As a result, oxygen will diffuse from outside the cell directly through the lipid bilayer of the membrane and into the cytoplasm within the cell. On the other hand, because cells produce CO2 as a byproduct of metabolism, CO2 concentrations rise within the cytoplasm; therefore, CO2 will move from the cell through the lipid bilayer and into the extracellular fluid, where its concentration is lower (Figure 4.3.1).
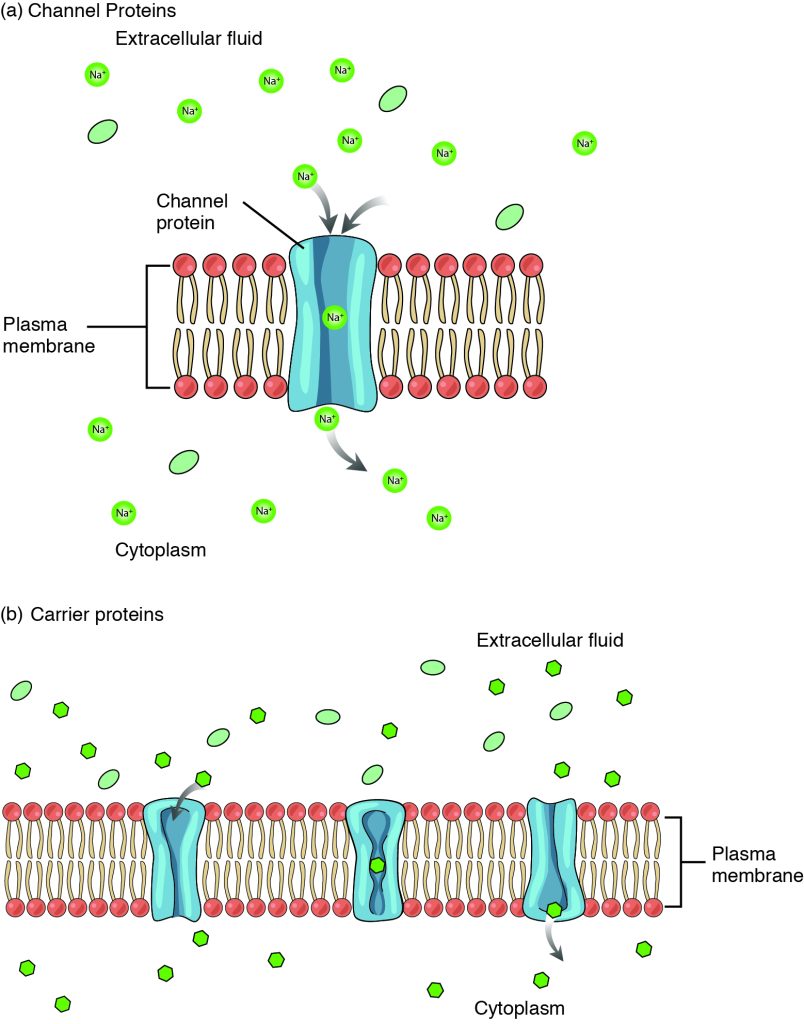
Large polar or ionic molecules, which are hydrophilic, cannot easily cross the phospholipid bilayer. Charged atoms or molecules of any size cannot cross the cell membrane via simple diffusion as the charges are repelled by the hydrophobic tails in the interior of the phospholipid bilayer. Solutes dissolved in water on either side of the cell membrane will tend to diffuse down their concentration gradients, but because most substances cannot pass freely through the lipid bilayer of the cell membrane, their movement is restricted to protein channels and specialized transport mechanisms in the membrane. Facilitated diffusion is the diffusion process used for those substances that cannot cross the lipid bilayer due to their size, charge, and/or polarity but do so down their concentration gradients (Figure 4.3.2). As an example, even though sodium ions (Na+) are highly concentrated outside of cells, these electrolytes are charged and cannot pass through the nonpolar lipid bilayer of the membrane. Their diffusion is facilitated by membrane proteins that form sodium channels (or “pores”), so that Na+ ions can move down their concentration gradient from outside the cells to inside the cells. A common example of facilitated diffusion using a carrier protein is the movement of glucose into the cell, where it is used to make ATP. Although glucose can be more concentrated outside of a cell, it cannot cross the lipid bilayer via simple diffusion because it is both large and polar, and therefore, repelled by the phospholipid membrane. To resolve this, when blood glucose levels are high, a specialized carrier protein called the GLUT4 glucose transporter is inserted into the plasma membrane of cells like skeletal muscle and adipocytes to transfer glucose molecules into the cell and facilitate its inward diffusion.
The difference between a channel and a carrier is that the carrier usually changes shape during the diffusion process, while the channel does not. There are many other solutes that must undergo facilitated diffusion to move into a cell, such as amino acids, or to move out of a cell, such as wastes.
Osmosis
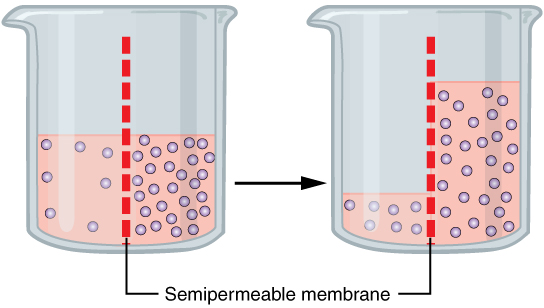
A specialized example of facilitated transport is water moving across the cell membrane of all cells, through protein channels known as aquaporins. Osmosis is the diffusion of water through a semipermeable membrane from where there is more relative water to where there is less relative water (down its water concentration gradient) (Figure 4.3.3). While water can move through a plasma membrane, it does so very slowly due to the hydrophobic fatty acid tails making up the core of the membrane. All cells have aquaporins, or water channels, embedded in the plasma membrane to speed up the movement of water into and out of cells. Most aquaporins are always open, allowing water to easily move from one side of the membrane to the other.
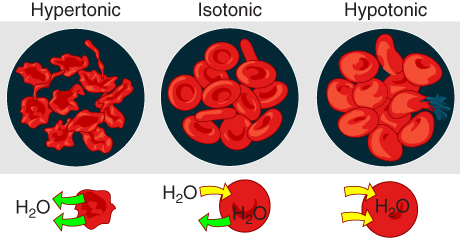
On their own, cells cannot regulate the movement of water molecules across their membrane, so it is important that cells are exposed to an environment in which the concentration of solutes outside of the cells (in the extracellular fluid) is equal to the concentration of solutes inside the cells (in the cytoplasm). Two solutions that have the same concentration of solutes are said to be isotonic (equal tension). When cells and their extracellular environments are isotonic, the concentration of water molecules is the same outside and inside the cells, and the cells maintain their normal shape (and function).
Osmosis occurs when there is an imbalance of solutes outside of a cell versus inside the cell. A solution that has a higher concentration of solutes than another solution is said to be hypertonic, and water molecules tend to diffuse into a hypertonic solution (Figure 4.3.4). Cells in a hypertonic solution will shrivel as water leaves the cell via osmosis. In contrast, a solution that has a lower concentration of solutes than another solution is said to be hypotonic, and water molecules tend to diffuse out of a hypotonic solution. Cells in a hypotonic solution will take on too much water and swell, with the risk of eventually bursting. A critical aspect of homeostasis in living things is to create an internal environment in which all of the body’s cells are in an isotonic solution. Various organ systems, particularly the kidneys, work to maintain this homeostasis.
Fluid Movement Between Compartments
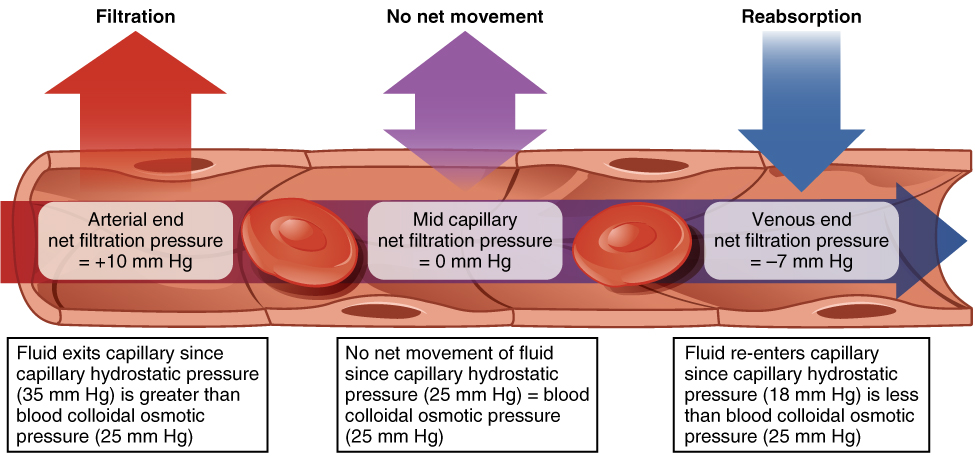
Hydrostatic pressure, the force exerted by a fluid against a wall, causes movement of fluid between compartments via filtration. The hydrostatic pressure of blood is the pressure exerted by blood against the walls of the blood vessels by the pumping action of the heart. In capillaries, hydrostatic pressure (also known as capillary blood pressure) is higher than the opposing colloid osmotic pressure in blood—a constant pressure drawing water back into the plasma that is primarily produced by circulating proteins—at the arteriolar end of the capillary (Figure 4.3.5). Filtration pressure squeezes fluid from the plasma in the blood to the interstitial fluid surrounding the tissue cells near the arterial end of a capillary bed. Fluid and the cellular wastes in the tissues enter the capillaries at the venous end of the capillary, where the hydrostatic pressure is less than the osmotic pressure in the vessel. Any surplus fluid in the interstitial space that is not returned directly back to the capillaries is drained from tissues by the lymphatic system and then reenters the vascular system at the subclavian veins.
External Website

Watch this video to see an explanation of the dynamics of fluid in the body’s compartments. What happens in the tissue when capillary blood pressure is less than osmotic pressure?
Hydrostatic pressure is especially important in governing the movement of water in the nephrons of the kidneys to ensure proper filtering of the blood to form urine. As hydrostatic pressure in the kidneys increases, the amount of water leaving the capillaries also increases, and more urine filtrate is formed. If hydrostatic pressure in the kidneys drops too low, as can happen in dehydration, the functions of the kidneys will be impaired, and less nitrogenous wastes will be removed from the bloodstream. Extreme dehydration can result in kidney failure.
Fluid also moves between intracellular fluid, interstitial fluid, and plasma along an osmotic gradient. The magnitude of the osmotic gradient is proportional to the difference in the concentration of solutes on one side of the cell membrane to that on the other side. In the body, water moves constantly into and out of fluid compartments as conditions change in different parts of the body. For example, if you are sweating, you will lose water through your skin. Sweating depletes your tissues of water and increases the solute concentration in those tissues. As this happens, water diffuses from your blood into sweat glands and surrounding skin tissues that have become dehydrated because of the osmotic gradient. Additionally, as water leaves the blood, it is replaced by the water in other tissues throughout your body that are not dehydrated. If this continues, dehydration spreads throughout the body. When a dehydrated person drinks water and rehydrates, the water is redistributed by the same gradient, but in the opposite direction, replenishing water in all of the tissues.
Disorders of Fluid Balance: Edema
Edema is the accumulation of excess water in the tissues. It is most common in the soft tissues of the extremities. The physiological causes of edema include water leakage from blood capillaries. Edema is almost always caused by an underlying medical condition, the use of certain therapeutic drugs, pregnancy, localized injury, or an allergic reaction. In the limbs, the symptoms of edema include swelling of the subcutaneous tissues; an increase in the normal size of the limb; and stretched, tight skin. One quick way to check for subcutaneous edema localized in a limb is to press a finger into the suspected area. Edema is likely if the depression persists for several seconds after the finger is removed (which is called “pitting”).
Pulmonary edema is excess fluid in the air sacs of the lungs, a common symptom of heart and/or kidney failure. People with pulmonary edema likely will experience difficulty breathing, and they may experience chest pain. Pulmonary edema can be life threatening because it compromises gas exchange in the lungs, and anyone having symptoms should immediately seek medical care.
In pulmonary edema resulting from heart failure, excessive leakage of water occurs because fluids get “backed up” in the pulmonary capillaries of the lungs, when the left ventricle of the heart is unable to pump sufficient blood into the systemic circulation. Because the left side of the heart is unable to pump out its normal volume of blood, the blood in the pulmonary circulation gets “backed up,” starting with the left atrium, then into the pulmonary veins, and then into pulmonary capillaries. The resulting increased hydrostatic pressure within pulmonary capillaries, as blood is still coming in from the pulmonary arteries, causes fluid to be pushed out of them and into lung tissues.
Other causes of edema include damage to blood vessels or lymphatic vessels or a decrease in osmotic pressure in chronic and severe liver disease, where the liver is unable to manufacture plasma proteins (Figure 4.3.6). A decrease in the normal levels of plasma proteins results in a decrease of colloid osmotic pressure (which counterbalances the hydrostatic pressure) in the capillaries. This process causes loss of water from the blood to the surrounding tissues, resulting in edema.

Mild, transient edema of the feet and legs may be caused by sitting or standing in the same position for long periods of time, as in the work of a toll collector or a supermarket cashier. This is because deep veins in the lower limbs rely on skeletal muscle contractions to push on the veins and thus “pump” blood back to the heart. Otherwise, the venous blood pools in the lower limbs and can leak into surrounding tissues.
Medications that can result in edema include vasodilators, calcium channel blockers used to treat hypertension, nonsteroidal anti-inflammatory drugs, estrogen therapies, and some diabetes medications. Underlying medical conditions that can contribute to edema include congestive heart failure, kidney damage and kidney disease, disorders that affect the veins of the legs, and cirrhosis and other liver disorders.
Therapy for edema usually focuses on elimination of the cause. Activities that can reduce the effects of the condition include appropriate exercises to keep the blood and lymph flowing through the affected areas. Other therapies include elevation of the affected part to assist drainage, massage and compression of the areas to move the fluid out of the tissues, and decreased salt intake to decrease sodium and water retention.
Section Review
Passive transport is the movement of substances across cell membranes without the expenditure of energy by the cell. It relies on concentration gradients and diffusion, where molecules move from areas of higher concentration to lower concentration. Simple diffusion allows small lipid-soluble molecules like oxygen and carbon dioxide to pass through the cell membrane, while facilitated diffusion requires protein channels or carriers for larger or charged molecules like glucose and sodium ions. Osmosis, the diffusion of water through aquaporins, is also crucial for maintaining isotonic conditions and ensuring cell health. Water and solutes can also move from one fluid compartment to another by filtration, where blood pressure within a vessel pushes fluid from the blood plasma into interstitial fluid.
Interactive Link Questions
Notice diffusion in the gif below, and how it is propelled by the kinetic energy of molecules in solution.

Watch this video to see an explanation of the dynamics of fluid in the body’s compartments.
Video 4.3. Fluids and Electrolytes Part 2 by Dr. John Campbell
Review Questions
Critical Thinking Questions
Glossary
- concentration gradient
- the difference in concentration of a substance across a space
- diffusion
- the movement of particles from an area of higher concentration to an area of lower concentration
- facilitated diffusion
- the diffusion process used for substances that cannot cross the lipid bilayer due to their size, charge, and/or polarity but do so down their concentration gradients
- homeostasis
- the process of maintaining a stable internal environment in living organisms
- hydrostatic pressure
- pressure exerted by a fluid against a wall, caused by its own weight or pumping force
- hypertonic
- a solution that has a higher concentration of solutes than another solution
- hypotonic
- a solution that has a lower concentration of solutes than another solution
- isotonic
- a solution that has the same concentration of solutes as another solution
- osmosis
- the diffusion of water through a semipermeable membrane from where there is more relative water to where there is less relative water
- passive transport
- the movement of molecules across a cell membrane without the cell expending energy
- simple diffusion
- a form of passive transport where molecules move across a cell membrane from the side where they are more concentrated to the side where they are less concentrated
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Image Descriptions
Figure 4.3.1. This illustration depicts the process of simple diffusion across a cell membrane over time. The diagram is divided into three sequential panels from left to right, with a “Time” arrow at the bottom indicating temporal progression. Each panel shows a cross-section of the “Lipid bilayer (plasma membrane)” (labeled on the left) represented as a phospholipid bilayer with orange/coral circles as heads and lines as tails. “Small uncharged molecules” (labeled at top left) are shown as light blue hexagons. The top region is labeled “Extracellular fluid” and the bottom region is labeled “Cytoplasm” (center panel). The sequence shows the molecules initially concentrated in the extracellular fluid (left panel), then beginning to move through the membrane (center panel), and finally distributing more evenly between both compartments (right panel), illustrating the passive movement of molecules down their concentration gradient through the lipid bilayer without requiring energy or membrane proteins. [Return to Figure 4.3.1]
Figure 4.3.2. This illustration compares two types of facilitated diffusion across cell membranes. Panel (a), labeled “Channel Proteins,” shows a cross-section of the “Plasma membrane” (phospholipid bilayer with red/coral circles as heads) with a blue “Channel protein” creating a pore through the membrane. Green circles labeled “Na⁺” (sodium ions) and green ovals (representing other small molecules) are shown in the “Extracellular fluid” (top) and “Cytoplasm” (bottom). Gray arrows indicate sodium ions moving through the channel pore down their concentration gradient. Panel (b), labeled “Carrier proteins,” shows a similar membrane cross-section with three blue carrier proteins embedded in the “Plasma membrane.” Green circles and ovals representing molecules are distributed in the “Extracellular fluid” (top) and “Cytoplasm” (bottom). The carrier proteins are shown in different conformations, with one displaying a gray arrow indicating the protein binding to a molecule and changing shape to transport it across the membrane. Both panels illustrate passive transport mechanisms that allow specific molecules to cross the membrane without energy expenditure. [Return to Figure 4.3.2]
Figure 4.3.3. This illustration depicts the process of osmosis using two glass beakers separated by time, connected by a black arrow indicating progression. Both beakers contain a “Semipermeable membrane” (labeled at bottom) shown as a vertical red dashed line dividing each beaker into two compartments. In the left beaker, the left compartment contains pale pink liquid with few small circles (low solute concentration) at a higher level, while the right compartment contains darker pink liquid with many small purple circles (high solute concentration) at a lower level. The right beaker shows the result after osmosis has occurred: the fluid levels have equalized somewhat, with water having moved from the left side to the right side across the semipermeable membrane, increasing the volume on the high-solute side while the solute particles (purple circles) remain concentrated on their original side. This demonstrates how water moves across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration to achieve equilibrium. [Return to Figure 4.3.3]
Figure 4.3.4. This illustration demonstrates osmotic effects on red blood cells in three different solution types. The top row shows three circular views labeled “Hypertonic,” “Isotonic,” and “Hypotonic” from left to right, each containing red blood cells against a dark background. In the hypertonic solution (left), the red blood cells appear shriveled and irregular with a crenated appearance. In the isotonic solution (center), the cells maintain their normal biconcave disc shape. In the hypotonic solution (right), the cells appear swollen and rounded. The bottom row shows simplified diagrams of water (H₂O) movement for each condition: under the hypertonic cell, green arrows point outward from the cell indicating water loss; under the isotonic cell, green arrows point both in and out indicating balanced water movement; under the hypotonic cell, yellow arrows point inward indicating water influx into the cell. This diagram illustrates how osmotic pressure differences cause water to move across cell membranes, affecting cell shape and volume. [Return to Figure 4.3.4]
Figure 4.3.5. This illustration depicts fluid exchange across a capillary wall at three different points. The diagram shows a cross-sectional view of a capillary with red blood cells flowing through it. The left section labeled “Filtration” shows a red upward arrow and displays “Arterial end net filtration pressure = +10 mm Hg” with explanatory text below stating “Fluid exits capillary since capillary hydrostatic pressure (35 mm Hg) is greater than blood colloidal osmotic pressure (25 mm Hg)”. The middle section labeled “No net movement” shows a purple bidirectional arrow and displays “Mid capillary net filtration pressure = 0 mm Hg” with text explaining “No net movement of fluid since capillary hydrostatic pressure (25 mm Hg) = blood colloidal osmotic pressure (25 mm Hg)”. The right section labeled “Reabsorption” shows a blue downward arrow and displays “Venous end net filtration pressure = –7 mm Hg” with text stating “Fluid re-enters capillary since capillary hydrostatic pressure (18 mm Hg) is less than blood colloidal osmotic pressure (25 mm Hg)”. The diagram illustrates how the balance between hydrostatic pressure and osmotic pressure determines the direction of fluid movement across the capillary wall. [Return to Figure 4.3.5]
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
