Chapter 4. Membranes and Transport
4.5 Membrane Potential
Learning Objectives
By the end of this section, you will be able to:
- Define resting membrane potential (RMP) of a cell
- Explain how RMP is measured and give a typical value for RMP
- Calculate the equilibrium potential for an ion
All animal cells are surrounded by a plasma membrane composed of a lipid bilayer that regulates the entry and exit of substances from the cell. Due to the action of ion pumps, symporters, antiporters, and channels, the composition of ions inside the cell is different from the composition outside the cell. This difference in ion composition forms the basis of a cell’s membrane potential, or the difference in charge inside versus outside the cell. The membrane potential has two basic functions. First, it allows a cell to function as a battery, providing power in the form of an electrochemical gradient to operate a variety of “molecular devices” embedded in the membrane. Second, in electrically excitable cells such as neurons and muscle cells, it is used for transmitting signals between different parts of a cell. Signals are generated by opening or closing of ion channels at one point in the membrane, producing a local change in the membrane potential that causes electric current to flow rapidly to other points in the membrane.
Membrane Potential
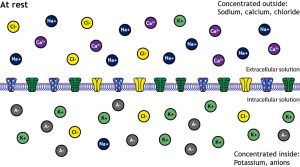
As discussed in Chapter 4.1, the composition of the intracellular fluid (ICF) and the extracellular fluid (ECF) surrounding the cell differs greatly in the concentration of different ions inside and outside the cell. Specifically, the ECF has higher concentrations of sodium (Na+), calcium (Ca2+), and chloride (Cl–), while the ICF has higher concentrations of potassium (K+) and fixed anions like proteins and nucleic acids (Figure 4.5.1).
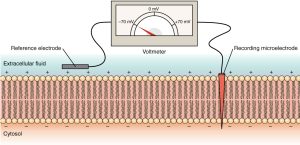
This difference in electrical charge between the interior and the exterior of a biological cell results in the cell’s membrane potential (also transmembrane potential or membrane voltage). A cell’s membrane potential can be directly measured using a voltmeter and two recording electrodes, one placed outside the cell and the other placed inside the cell. Membrane potential can then be determined by comparing the charge inside the cell to the charge outside the cell (Figure 4.5.2).
Voltage is the measure of potential energy generated by separated charges. The difference in charge between the interior and exterior of the cell is so small, membrane potential is typically measured in millivolts (1 volt = 1,000 millivolts). The greater the difference in charge between two points, the higher the voltage. In non-excitable cells, and in excitable cells in their baseline states, the membrane potential is held at a relatively stable value called the resting membrane potential (RMP) or resting potential.
For neurons, typical values of the resting potential range from –70 to –80 millivolts (mV); that is, the interior of a cell has a negative baseline voltage of a bit less than one tenth of a volt. The opening and closing of ion channels can induce a departure from the resting potential. This is called a depolarization if the interior voltage becomes more positive (say from –70 mV to –60 mV) or a hyperpolarization if the interior voltage becomes more negative (say from –70 mV to –80 mV). The changes in membrane potential can be small or larger (graded potentials) depending on how many ion channels are activated and what type they are. The ability of neurons to manipulate membrane potential is discussed in greater detail in Chapter 5.
Equilibrium Potential
Each ion is subject to its electrochemical gradient, which is a combination of the concentration gradient for that ion and the attraction or repulsion that ion has to each side of the membrane. For example, the concentration of Na+ outside of cells is roughly 10x higher than the concentration of Na+ inside the cell, resulting in a steep concentration gradient that favors the movement of Na+ into the cell. In addition, Na+ is positively charged while the interior of the cell has a slight negative charge; positive charges are attracted to negative charges, so this additional force will drive Na+ into the cell as well. However, if the interior of the cell becomes positively charged, the repulsion of like charges will slow or even halt the flow of Na+ into the cell.
The membrane potential of the cell at which there is no net movement for a given ion is called the equilibrium potential for that ion. When the equilibrium potential is reached, the concentration gradient and electrical gradient for that ion are in balance such that the ion is not drawn to one side of the membrane or the other. The equilibrium potential for a given ion can be calculated using the Nernst equation, named after German physicist Walther Nernst, who derived this equation in 1887. The simplified form of the equation is as follows:
![]()
Eion = the equilibrium potential for a given ion, in mV
61 = constant representing the universal gas constant, temperature, and Faraday’s constant
z = the valence (total charge) of the ion
[ion]out = the concentration of the ion outside the cell
[ion]in = the concentration of the ion inside the cell
Using Na+ as an example, Na+ has an extracellular concentration of 145 millimolar (mM), an intracellular concentration of 12 mM, and a valence of +1. With these values, the equilibrium potential for sodium is as follows:
![]()
If the membrane is permeable, an ion will move across the cell membrane to bring the membrane potential closer to its equilibrium. This means that if a cell had a resting potential of -70 mV and Na+ channels were to open, Na+ ions would rush into the cell; the positive charges entering would make the interior of the cell more positive, bringing the membrane potential closer to +66 mV. In order to stop Na+ from entering the cell, the membrane potential would have to be +66 mV. If the membrane potential exceeded +66 mV, Na+ would exit the cell to reach its equilibrium potential.
Potassium has very different conditions. For a typical cell, the extracellular concentration of K+ is 5 mM and the intracellular concentration is 155 mM. With these values, the equilibrium potential for K+ is as follows:
![]()
This means that if the cell was at rest with a membrane potential of -70 mV and K+ were to open, K+ would exit the cell; the movement of positive charges out of the cell leaves the cell more negatively charged than it was before, which would drive the membrane potential closer to -90 mV.
Membrane Potential and Ion Flow
While there are many charged substances inside and outside a cell that contribute to the resting membrane potential, two ions in particular play a major role in establishing and maintaining the resting membrane potential: Na+ and K+. Even a cell at rest will have some Na+ moving into the cell, either through random opening of Na+ leakage channels or as a consequence of secondary active transport. Many cells also have dedicated leakage channels for K+ that allow K+ to leak out of the cell as well. In general, the presence of K+ leakage channels means that the membrane is typically more permeable to K+ than to Na+. For this reason, the resting membrane potential is typically close to the equilibrium potential for K+.
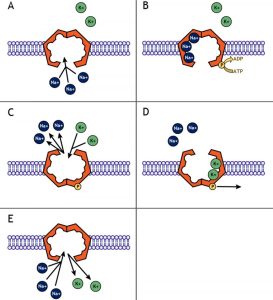
In order to counteract the movement of these cations and maintain a stable resting membrane potential, the sodium-potassium pump functions to maintain the concentration gradients of these ions. The pump moves three Na+ ions out of the cell and returns two K+ ions into the cell for every ATP consumed (Figure 4.5.3). This pushes more cations out of the cell than are brought in, keeping the interior of the cell more negative relative to the outside environment. Since K+ is being returned to the interior of the cell against its gradient, the resting potential is not equal to the equilibrium potential for K+ but is slightly more positive at around -70 mV.
Section Review
Animal cells maintain a membrane potential—a voltage difference across the plasma membrane—through the differential distribution of ions such as sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) between the intracellular and extracellular environments. This potential arises from the coordinated activity of ion pumps, channels, and transporters that create electrochemical gradients. The membrane potential enables the cell to function like a battery, powering various cellular processes, and in excitable cells like neurons and muscle fibers, it facilitates rapid signal transmission. The resting membrane potential, typically around –70 mV in neurons, reflects a dynamic equilibrium influenced especially by K⁺ and Na⁺ ions, with K⁺ playing a dominant role due to greater membrane permeability. Each ion has an equilibrium potential—the voltage at which its net movement across the membrane stops—determined by its concentration gradient and charge, as calculated by the Nernst equation. The sodium-potassium pump maintains these gradients by exporting three Na⁺ ions and importing two K⁺ ions per ATP molecule used, preserving the cell’s negative interior and ensuring the resting membrane potential stays near, but slightly above, K⁺’s equilibrium potential.
Review Questions
Critical Thinking Questions
Glossary
- anion
- a negatively charged ion
- cation
- a positively charged ion
- depolarization
- a change in membrane potential making the inside of the cell more positive than at rest
- electrochemical gradient
- the combined effect of an ion’s concentration gradient and the electrical charge gradient across a membrane
- equilibrium potential
- the membrane voltage at which there is no net movement of a particular ion across the membrane
- hyperpolarization
- a change in membrane potential that makes the interior of the cell more negative than at rest
- leakage channel
- an ion channel that is always open, allowing ions to move according to their gradients
- membrane potential
- the electrical potential difference across the plasma membrane due to ion distribution
- Nernst equation
- an equation used to calculate the equilibrium potential of an ion based on its concentration gradient and charge
- resting membrane potential
- the stable membrane voltage of a non-excited cell, typically around –70 mV
- voltage
- the measure of potential energy caused by separated charges across a membrane
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY, and A and P for STEM Educators by LibreText, licensed under CC BY-SA. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Image Descriptions
Figure 4.5.1. This educational illustration depicts the ionic distribution across a cell membrane at rest. The diagram is divided into two sections by a horizontal plasma membrane (shown as a purple phospholipid bilayer with embedded proteins in the center). The top section labeled “At rest” and “Concentrated outside” shows the extracellular space with various colored circles representing different ions: dark blue circles marked with minus signs (negative ions), yellow circles with plus signs (positive ions), purple circles with minus signs, and green circles with minus signs distributed above the membrane. The bottom section labeled “Concentrated inside” shows the intracellular space with similar colored circles but in different concentrations. Text labels indicate “Extracellular volume (ECF)” on the right side of the upper section and “Intracellular volume (ICF)” implied for the lower section. The distribution pattern shows a higher concentration of negative charges inside the cell and positive charges outside, illustrating the resting membrane potential and electrochemical gradients that exist across the plasma membrane. The membrane contains various channel proteins shown as gray and colored structures embedded in the lipid bilayer. [Return to Figure 4.5.1]
Figure 4.5.2. This educational illustration depicts the patch clamp technique used to measure ion channel activity in cell membranes. The diagram shows a cross-section with labeled components: at the top, a recording device displays electrical trace readings; a “Recording micropipette” (labeled on right) extends down through the “Extracellular fluid” (labeled on left) to form a tight seal with the cell membrane. The membrane is shown as a pink/coral phospholipid bilayer with individual lipid molecules visible. A “Reference electrode” (labeled on left) is positioned in the extracellular fluid, while the recording micropipette creates a seal on a small patch of membrane containing ion channels. The bottom portion shows the “Cytosol” (labeled on left) beneath the membrane. This technique allows researchers to measure electrical currents flowing through individual ion channels by isolating a small section of membrane and recording voltage changes or current flow with high precision. [Return to Figure 4.5.2]
Figure 4.5.3. This educational illustration depicts the five stages of receptor-mediated endocytosis across panels labeled A through E. Each panel shows a cross-section of the purple phospholipid bilayer cell membrane. Panel A shows green circles labeled “Ligand” above the membrane and dark blue circles below, with a red/brown curved receptor protein spanning the membrane. Panel B shows the ligands binding to the receptor protein, which begins to change conformation. Panel C shows the membrane beginning to invaginate (curve inward) as more ligands bind to clustered receptors, with the dark blue circles (representing coat proteins) beginning to assemble on the cytoplasmic side. Panel D shows further invagination with the forming vesicle nearly pinched off from the membrane. Panel E shows the completed process with a fully formed coated vesicle in the cytoplasm containing the bound ligands and receptors, now separated from the plasma membrane. This diagram illustrates how cells selectively internalize specific molecules by using membrane receptors and clathrin-coated vesicles. [Return to Figure 4.5.3]
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
