Chapter 8. Muscle Tissue
8.3 Muscle Fiber Excitation, Contraction, and Relaxation
Learning Objectives
By the end of this section, you will be able to:
- Diagram and describe the structure of a neuromuscular junction (NMJ) between a motor neuron and a skeletal muscle fiber (cell)
- Describe the events involved in the contraction and relaxation of a skeletal muscle fiber (cell), including events at the neuromuscular junction, excitation-contraction coupling, and the process of cross-bridge cycling
- Explain the role of adenosine triphosphate (ATP)/adenosine diphosphate (ADP) and myosin ATPase in the contraction cycle and the rigor state
- Explain the importance of glycogen, creatine kinase, and phosphocreatine for ATP production in striated muscles
- Compare and contrast aerobic versus anaerobic ATP production, including the advantages and disadvantages of both
- Define muscular fatigue and explain the factors that may contribute to skeletal muscle fatigue
- Define and explain excess post-exercise oxygen consumption (EPOC), including how it relates to ATP production in working muscles
- Outline the physiological basis of Duchenne muscular dystrophy (DMD) and identify the demographic most commonly affected
The Neuromuscular Junction
The process of muscle contraction begins at the site where a motor neuron’s terminal meets the muscle fiber—called the neuromuscular junction (NMJ). This is where the muscle fiber first responds to signaling by the motor neuron. Every skeletal muscle fiber in every skeletal muscle is innervated by a motor neuron at a NMJ. Excitation signals from the motor neuron are the only way to functionally activate skeletal muscle fibers to contract.
External Website

Every skeletal muscle fiber is supplied by a motor neuron at the NMJ. Watch this video to learn more about what happens at the NMJ.
Excitation-Contraction Coupling
All living cells have membrane potentials, or electrical gradients across their membranes based on the distribution of positively and negatively charged ions. The inside of the membrane is usually around -60 to -90 millivolts (mV), relative to the outside. Neurons and muscle cells can use their membrane potentials to generate and conduct electrical signals by controlling the movement of charged ions across their membranes to create electrical currents. This movement is controlled by selective opening and closing of specialized proteins in the membrane called ion channels. Although the currents generated by ions moving through these channel proteins are very small, they form the basis of both neural signaling and muscle contraction.
Both neurons and skeletal muscle cells are electrically excitable, meaning that they are able to generate action potentials. An action potential is a special type of electrical signal that can travel along a cell membrane as a wave. This allows a signal to be transmitted quickly over long distances.
The term excitation-contraction coupling refers to the fact that for a skeletal muscle fiber to contract, its membrane must first be “excited”—in other words, it must be stimulated to fire an action potential. The muscle fiber action potential, which sweeps along the sarcolemma as a wave, is “coupled” to the actual contraction process through the release of calcium ions (Ca++) from the sarcoplasmic reticulum (SR). Once released, the Ca++ interacts with the shielding proteins (troponin and tropomyosin) on the actin thin filament, forcing them to move aside so that the binding sites are available for attachment by myosin heads. The myosin heads then pull the actin filaments toward the center of each sarcomere, shortening the overall muscle fiber.
In skeletal muscle, this sequence begins with signals from the somatic motor division of the nervous system. In other words, the “excitation” step in skeletal muscles is always triggered by signaling from the nervous system (Figure 8.3.1).
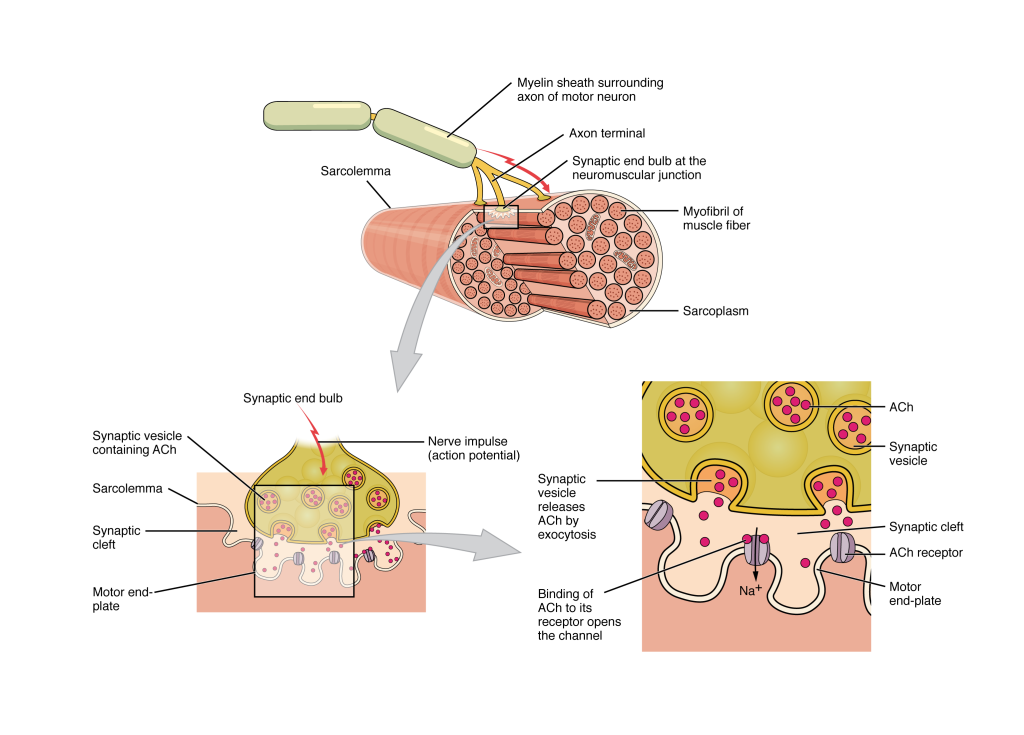
Most motor neurons that tell the skeletal muscle fibers to contract originate in the spinal cord. A smaller number of motor neurons are located in the brain stem for activation of skeletal muscles of the face, head, and neck. These neurons have long processes, called axons, which are specialized to transmit action potentials long distances—in this case, all the way from the spinal cord to the muscle itself (which may be up to three feet away). The axons of multiple neurons bundle together to form nerves, like wires bundled together in a cable.
Signaling begins when a neuronal action potential travels along the axon of a motor neuron and then along the individual branches to terminate at the NMJ. At the NMJ, the axon terminal releases a chemical messenger, or neurotransmitter, called acetylcholine (ACh). The ACh molecules diffuse across a minute space called the synaptic cleft and bind to ACh receptors located within the motor end-plate of the sarcolemma on the other side of the synapse. Once ACh binds, a channel in the ACh receptor opens and positively charged ions can pass through into the muscle fiber, causing it to depolarize, meaning that the membrane potential of the muscle fiber becomes less negative (closer to zero).
As the membrane depolarizes, another set of ion channels called voltage-gated sodium channels are triggered to open. Sodium ions enter the muscle fiber, and an action potential rapidly spreads (or “fires”) along the entire membrane of the muscle to initiate excitation-contraction coupling.
Things happen very quickly in the world of excitable membranes (just think about how quickly you can snap your fingers as soon as you decide to do it). Immediately following depolarization of the membrane, repolarization occurs, re-establishing the negative membrane potential. Meanwhile, the ACh in the synaptic cleft is degraded by the enzyme acetylcholinesterase (AChE) so that the ACh cannot rebind to a receptor and reopen its channel, causing unwanted extended muscle excitation and contraction.
Propagation of an action potential along the sarcolemma is the excitation portion of excitation-contraction coupling and must be coupled to the release of calcium ions for contraction to occur. High concentrations of calcium in skeletal muscle are stored in the SR, which is a specialized type of smooth endoplasmic reticulum. Recall that the excitation discussed here actually triggers the release of calcium ions (Ca++) from its storage in the cell’s SR. For the action potential to reach the membrane of the SR, there are periodic invaginations in the sarcolemma, called T-tubules (“T” stands for “transverse”). Because the diameter of a muscle fiber can be up to 100 micrometers (μm), T-tubules ensure that action potentials can travel deep into muscle fibers and reach the SR associated with each myofibril. The arrangement of a T-tubule with the membranes of SR on either side is called a triad (Figure 8.3.2). The triad surrounds the cylindrical myofibrils, which contain actin and myosin.
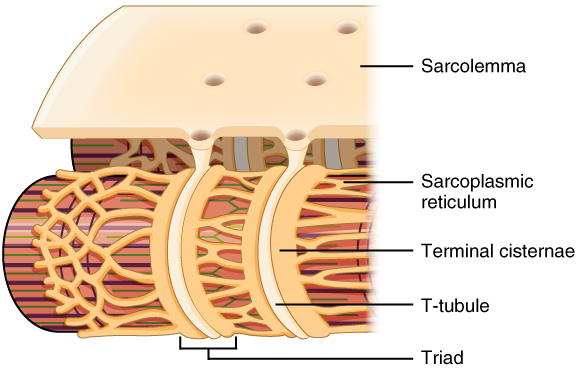
The T-tubules carry the action potential into the interior of the cell, which triggers the opening of calcium channels in the membrane of the adjacent SR, causing Ca++ to diffuse out of the SR and into the sarcoplasm. More specifically, voltage-sensitive dihydropyridine receptors (DHPR) on the sarcolemma are mechanically linked to calcium channels in the adjacent SR membrane called ryanadine receptors (RyR). Through the action of DHPR, an action potential in the sarcolemma triggers the opening of RyR, allowing Ca++ to diffuse out of the SR and into the sarcoplasm. It is the arrival of Ca++ in the sarcoplasm that initiates contraction of the muscle fiber by its contractile units, or sarcomeres.
Contraction of Skeletal Muscle
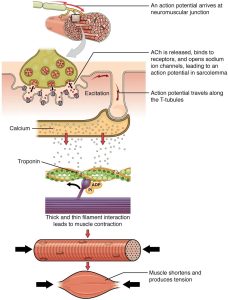
The sequence of events that result in the contraction of an individual muscle fiber begins with a signal—the neurotransmitter ACh—from the motor neuron innervating that fiber. The local membrane of the fiber will depolarize as positively charged sodium ions (Na+) enter, triggering an action potential that spreads to the rest of the membrane will depolarize, including the T-tubules. This triggers the release of calcium ions (Ca++) from storage in the SR. The Ca++ then initiates contraction, which is sustained by ATP (Figure 8.3.3). As long as Ca++ ions remain in the sarcoplasm to bind to troponin, which keeps the actin-binding sites “unshielded,” and as long as ATP is available to drive cross-bridge cycling (see following section), the muscle fiber will continue to shorten to an anatomical limit.
Cross-Bridge Cycling
As you have learned, during contraction the myosin heads of the thick filament bind to actin and pull the thin filament, which shortens the sarcomere and produces force. However, the length of the myosin hinge region allows each myosin head to only pull a very short distance before it must reset to pull again. For thin filaments to continue to slide past thick filaments during muscle contraction, myosin heads must pull the actin at the binding sites, detach, re-cock, attach to more binding sites, pull, detach, re-cock, and so on. This repeated movement is known cross-bridge cycling. This motion of the myosin heads is similar to the oars when an individual rows a boat: the paddle of the oars (the myosin heads) pull, are lifted from the water (detach), are repositioned (re-cocked) and then are immersed again to pull (Figure 8.3.4). Each cycle requires energy, and the action of the myosin heads in the sarcomeres repetitively pulling on the thin filaments also requires energy, which is provided by ATP.
Cross-bridge formation occurs when the myosin head attaches to the actin while adenosine diphosphate (ADP) and inorganic phosphate (Pi) are still bound to myosin (Figure 8.3.4a,b). Pi is then released, causing myosin to form a stronger attachment to the actin, after which the myosin head moves toward the M-line, pulling the actin along with it. As actin is pulled, the filaments move approximately 10 nanometers (nm) toward the M-line. This movement is called the power stroke, as movement of the thin filament occurs at this step (Figure 8.3.4c). In the absence of ATP, the myosin head will not detach from actin.
One part of the myosin head attaches to the binding site on the actin, but the head has another binding site for ATP. ATP binding causes the myosin head to detach from the actin (Figure 8.3.4d). After this occurs, ATP is converted to ADP and Pi by the intrinsic ATPase activity of myosin. The energy released during ATP hydrolysis changes the angle of the myosin head into a cocked position (Figure 8.3.4e). The myosin head is now in position for further movement.
When the myosin head is cocked, myosin is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke, and at the end of the power stroke, the myosin head is in a low-energy position. After the power stroke, ADP is released; however, the formed cross-bridge is still in place, and actin and myosin are bound together. As long as ATP is available, it readily attaches to myosin, the cross-bridge cycle can recur, and muscle contraction can continue.
Note that each thick filament of roughly 300 myosin molecules has multiple myosin heads, and many cross-bridges form and break continuously during muscle contraction. Multiply this by all of the sarcomeres in one myofibril, all the myofibrils in one muscle fiber, and all of the muscle fibers in one skeletal muscle, and you can understand why so much energy (ATP) is needed to keep skeletal muscles working. In fact, it is the loss of ATP that results in the rigor mortis observed soon after someone dies. With no further ATP production possible, there is no ATP available for myosin heads to detach from the actin-binding sites, so the cross-bridges stay in place, causing rigidity in the skeletal muscles.
Relaxation of Skeletal Muscle
Muscle contraction usually stops when signaling from the motor neuron ends, which stops it from releasing its chemical signal, ACh, into the synapse at the NMJ. Within the synapse AChE degrades any remaining ACh to prevent further stimulation. The muscle fiber repolarizes, which closes the gates in the SR where Ca++ was being released. ATP-driven pumps then move Ca++ out of the sarcoplasm and back into the SR (Figure 8.3.3). This results in the “reshielding” of the actin-binding sites on the thin filaments, as tropomyosin rolls back over the actin-binding sites in the absence of Ca++. Without the ability to form cross-bridge formation between the thin and thick filaments, the muscle fiber loses its tension and relaxes. A muscle also can stop contracting when it runs out of ATP and becomes fatigued.
External Website

The release of calcium ions initiates muscle contractions. Watch this video to learn more about the role of calcium.
Sources of ATP
ATP supplies the energy for muscle contraction to take place. In addition to its direct role in the cross-bridge cycle, ATP also provides the energy for the active-transport Ca++ pumps in the SR. Muscle contraction does not occur without sufficient amounts of ATP. The amount of ATP stored in muscle is very low, only sufficient to power a few seconds worth of contractions. As it is broken down, ATP must therefore be regenerated and replaced quickly to allow for sustained contraction. There are three mechanisms by which ATP can be regenerated in muscle cells: creatine phosphate metabolism, anaerobic glycolysis, and aerobic respiration.
Creatine phosphate is a molecule that can store energy in its phosphate bonds. In a resting muscle, excess ATP transfers its energy to creatine, producing ADP and creatine phosphate. This acts as an energy reserve that can be used to quickly create more ATP. When the muscle starts to contract and needs energy, creatine phosphate transfers its phosphate back to ADP to form ATP and creatine. This reaction is catalyzed by the enzyme creatine kinase and occurs very quickly; thus, creatine phosphate-derived ATP powers the first few seconds of muscle contraction. However, creatine phosphate can only provide approximately 15 seconds worth of energy, at which point another energy source has to be used (Figure 8.3.5).
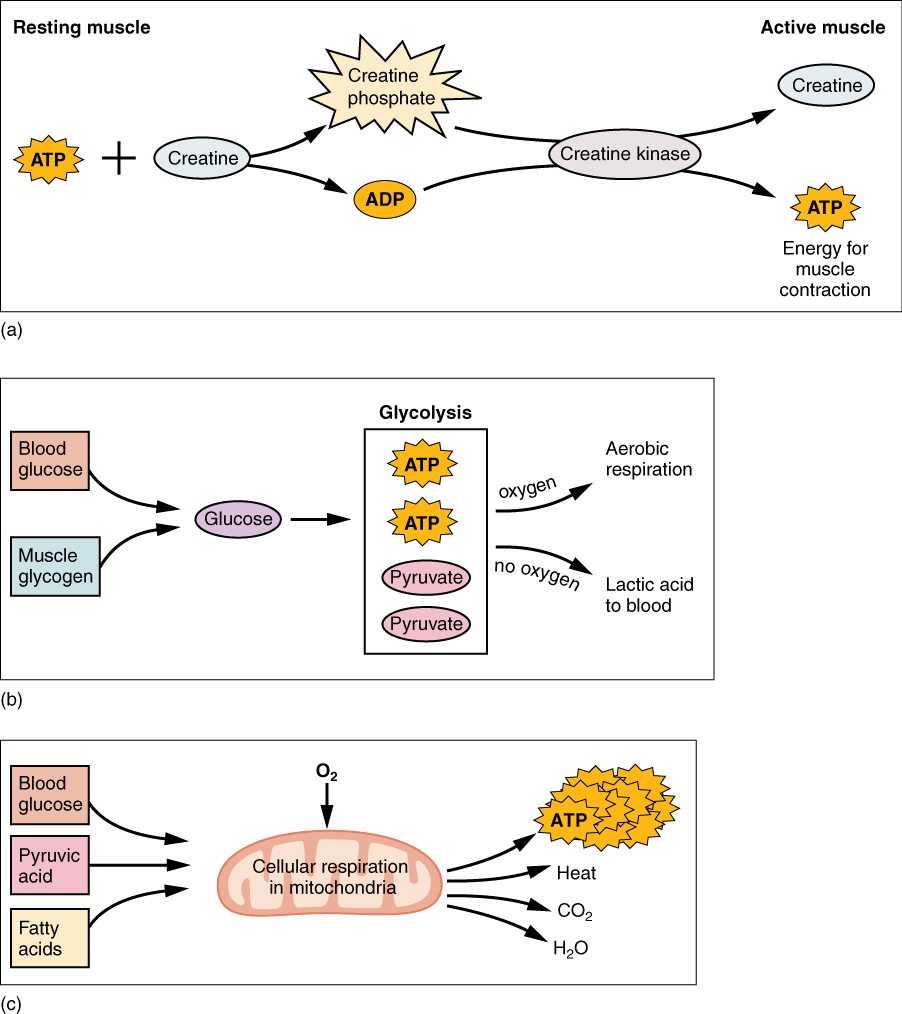
As the ATP produced by creatine phosphate is depleted, muscles turn to glycolysis as an ATP source. Glycolysis is an anaerobic (non-oxygen-dependent) process that breaks down glucose (sugar) to produce ATP; however, glycolysis cannot generate ATP as quickly as creatine phosphate. Thus, the switch to glycolysis results in a slower rate of ATP availability to the muscle. The sugar used in glycolysis can be provided by blood glucose or by metabolizing glycogen that is stored in the muscle. The breakdown of one glucose molecule produces two ATP and two molecules of pyruvic acid (pyruvate), which can be used in aerobic respiration or when oxygen levels are low, converted to lactic acid (Figure 8.3.5b).
If oxygen is available, pyruvic acid is used in aerobic respiration. However, if oxygen is not available, pyruvic acid is converted to lactic acid (lactate), which may contribute to muscle fatigue. This conversion allows the recycling of the enzyme NAD+ from NADH, which is needed for glycolysis to continue. This occurs during strenuous exercise when high amounts of energy are needed but oxygen cannot be sufficiently delivered to muscle. Glycolysis itself cannot be sustained for very long (approximately one minute of muscle activity), but it is useful in facilitating short bursts of high-intensity output. This is because glycolysis does not utilize glucose very efficiently, producing a net gain of two ATPs per molecule of glucose, and the end product of lactic acid, which may contribute to muscle fatigue as it accumulates.
Aerobic respiration is the breakdown of glucose or other nutrients in the presence of oxygen (O2) to produce carbon dioxide, water, and ATP. Approximately 95% of the ATP required for resting or moderately active muscles is provided by aerobic respiration, which takes place in mitochondria. The inputs for aerobic respiration include glucose circulating in the bloodstream, pyruvic acid, and fatty acids. Aerobic respiration is much more efficient than anaerobic glycolysis, producing approximately 36 ATPs per molecule of glucose versus four from glycolysis. However, aerobic respiration cannot be sustained without a steady supply of O2 to the skeletal muscle and is much slower (Figure 8.3.5c). To compensate, muscles store a small amount of oxygen locally in proteins called myoglobin, allowing for more efficient muscle contractions and less fatigue. Aerobic training also increases the efficiency of the circulatory system so that O2 can be supplied to the muscles for longer periods of time.
Muscle fatigue occurs when a muscle can no longer contract in response to signals from the nervous system. The exact causes of muscle fatigue are not fully known, although certain factors have been correlated with the decreased muscle contraction that occurs during fatigue. ATP is needed for normal muscle contraction, and as ATP reserves are reduced, muscle function may decline. This may be more of a factor in brief, intense muscle output rather than sustained, lower-intensity efforts. Lactic acid buildup may lower intracellular pH, affecting enzyme and protein activity. Additionally, accumulation of inorganic phosphate from ATP hydrolysis can influence fatigue. Imbalances in Na+ and K+ levels as a result of membrane depolarization may disrupt Ca++ flow out of the SR. Long periods of sustained exercise may damage the SR and the sarcolemma, resulting in impaired Ca++ regulation. A reduction in the number of motor units being activated or the firing frequency of those motor units may also lead to a reduced muscle force output.
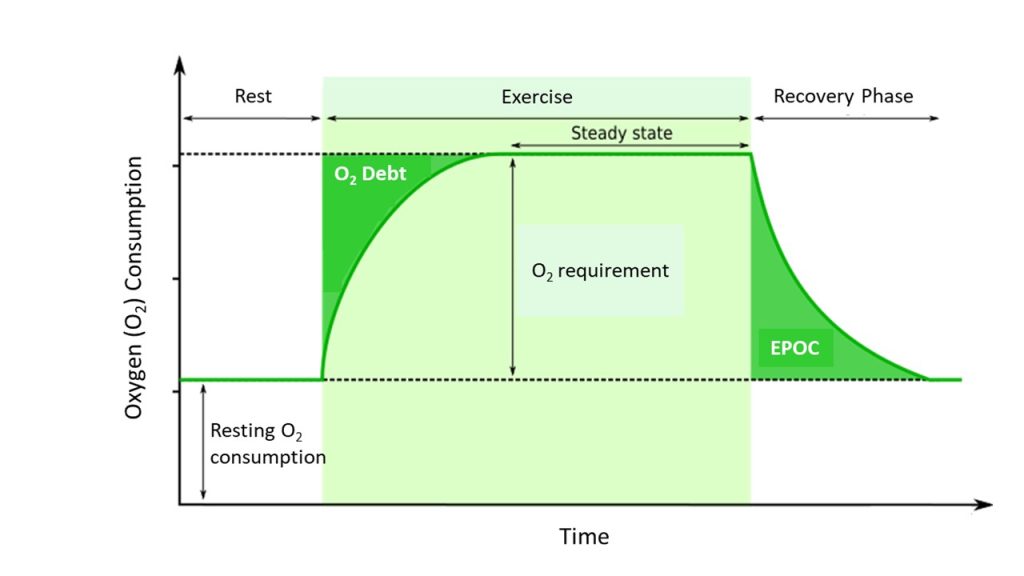
Intense muscle activity results in an oxygen debt, which is the amount of oxygen needed to compensate for ATP produced without oxygen during muscle contraction (Figure 8.3.6). When muscle activity increases and/or exercise is started, muscles may not be receiving adequate oxygen to produce the amount of ATP they need. During this time, anaerobic means are used to generate ATP while aerobic metabolism ramps up to meet the demand. As exercise continues, oxygen availability to the muscle is improved and the muscle moves into a steady state where oxygen consumption meets oxygen demand, and as a result, oxygen consumption plateaus. Once the activity is over, the body still requires elevated oxygen consumption to restore ATP and creatine phosphate levels, convert lactate back to pyruvate, and convert lactate into glucose and glycogen in the liver. Elevated levels of circulating hormones also keep the heart rate high, requiring additional oxygen. This results in the increased breathing rate that occurs after exercise. Until the oxygen debt has been met and the other perturbations from exercise are resolved, oxygen intake remains elevated, even after exercise has stopped. This is termed excess post-exercise oxygen consumption (EPOC).
Muscle Strength
Disorders of the Muscular System
Duchenne muscular dystrophy (DMD) is a progressive weakening of the skeletal muscles. It is one of several diseases collectively referred to as “muscular dystrophy.” DMD is caused by a lack of the protein dystrophin, which helps the thin filaments of myofibrils bind to the sarcolemma. Without sufficient dystrophin, muscle contractions cause the sarcolemma to tear, causing an influx of Ca++, leading to cellular damage and muscle fiber degradation. Over time, as muscle damage accumulates, muscle mass is lost and greater functional impairments develop.
DMD is an inherited disorder caused by an abnormal X chromosome. It primarily affects males, and it is usually diagnosed in early childhood. DMD usually first appears as difficulty with balance and motion and then progresses to an inability to walk. It continues progressing upward in the body from the lower extremities to the upper body, where it affects the muscles responsible for breathing and circulation. It ultimately causes death due to respiratory failure, and those afflicted do not usually live past their twenties.
Because DMD is caused by a mutation in the gene that codes for dystrophin, it was thought that introducing healthy myoblasts into patients might be an effective treatment. Myoblasts are the embryonic cells responsible for muscle development, and ideally, they would carry healthy genes that could produce the dystrophin needed for normal muscle contraction. This approach has been largely unsuccessful in humans. A recent approach has involved attempting to boost the muscle’s production of utrophin, a protein similar to dystrophin that may be able to assume the role of dystrophin and prevent cellular damage from occurring.
Section Review
The sequence of events that result in the contraction of an individual muscle fiber begins with a signal—the neurotransmitter ACh—from the motor neuron innervating that fiber at a neuromuscular junction (NMJ). The local membrane of the fiber will depolarize as positively charged sodium ions (Na+) enter, triggering an action potential that spreads to the rest of the membrane and deep into the muscle via T-tubules. This triggers the release of calcium ions (Ca++) from storage in the sarcoplasmic reticulum (SR). The Ca++ then binds to troponin, which moves tropomyosin off of myosin binding sites on actin, allowing cross-bridge formation. As long as Ca++ ions remain in the sarcoplasm and as long as ATP is available to drive cross-bridge cycling, the muscle fiber will continue to shorten to an anatomical limit. Ultimately, the shortening of muscle fibers produces movement.
There are three mechanisms by which ATP can be regenerated in muscle cells: creatine phosphate metabolism, anaerobic glycolysis, and aerobic respiration. Each can be used for various lengths of time and may be used to different degrees depending on the specific needs of a muscle. Muscle fatigue occurs when a muscle can no longer contract in response to signals from the nervous system.
Intense muscle activity results in an oxygen debt, which is the amount of oxygen needed to compensate for ATP produced without oxygen during muscle contraction. Until the oxygen debt has been met, oxygen intake will remain elevated even after exercise has stopped; this is known as excess post-exercise oxygen consumption (EPOC).
Review Questions
Critical Thinking Questions
Glossary
- acetylcholine (ACh)
- neurotransmitter that binds at a motor end-plate to trigger depolarization
- acetylcholinesterase (AChE)
- enzyme in the synaptic cleft that degrades ACh
- action potential
- change in voltage of a cell membrane in response to a stimulus that results in transmission of an electrical signal; unique to neurons and muscle fibers
- aerobic respiration
- production of ATP in the presence of oxygen
- ATPase
- enzyme that hydrolyzes ATP to ADP
- creatine phosphate
- phosphagen used to store energy from ATP and transfer it to muscle
- creatine kinase
- enzyme that catalyzes the reaction by which creatine phosphate transfers a phosphate back to ADP to form ATP and creatine
- depolarize
- to reduce the voltage difference between the inside and outside of a cell’s plasma membrane (the sarcolemma for a muscle fiber), making the inside less negative than at rest
- excess post-exercise oxygen consumption (EPOC)
- elevated oxygen consumption present at the end of an exercise bout that remains elevated until the oxygen debt is met and other disruptions to homeostasis have been rectified
- excitation-contraction coupling
- sequence of events from motor neuron signaling to a skeletal muscle fiber to contraction of the fiber’s sarcomeres
- glycolysis
- anaerobic breakdown of glucose to ATP
- lactic acid (lactate)
- product of anaerobic glycolysis
- motor end-plate
- sarcolemma of muscle fiber at the neuromuscular junction, with receptors for the neurotransmitter acetylcholine
- myoglobin
- oxygen-storing protein in muscle
- neuromuscular junction (NMJ)
- synapse between the axon terminal of a motor neuron and the section of the membrane of a muscle fiber with receptors for the acetylcholine released by the terminal
- neurotransmitter
- signaling chemical released by nerve terminals that bind to and activate receptors on target cells
- oxygen debt
- amount of oxygen needed to compensate for ATP produced without oxygen during muscle contraction
- power stroke
- action of myosin pulling actin inward (toward the M-line)
- pyruvic acid (pyruvate)
- product of glycolysis that can be used in aerobic respiration or converted to lactate
- repolarization
- re-establishment of negative membrane potential
- rigor mortis
- rigidity in the skeletal muscles shortly after death that results from a lack of ATP and inability for myosin heads to detach from actin-binding sites
- synaptic cleft
- space between a nerve (axon) terminal and a motor end-plate
- T-tubule
- projection of the sarcolemma into the interior of the cell
- triad
- the grouping of one T-tubule and two terminal cisternae
- voltage-gated sodium channels
- membrane proteins that open sodium channels in response to a sufficient voltage change and initiate and transmit the action potential as Na+ enters through the channel
Glossary Flashcards
This work, Human Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images from Anatomy & Physiology by OpenStax are licensed under CC BY except where otherwise noted.
Access the original for free at OpenStax.
Image Descriptions
Figure 8.3.1. This detailed anatomical diagram illustrates the neuromuscular junction, where a motor neuron communicates with a muscle fiber. The top section shows the axon of a motor neuron wrapped in a myelin sheath (depicted in pale yellow-green) approaching a muscle fiber. The axon terminal forms a synaptic end bulb at the neuromuscular junction, positioned above the sarcolemma (muscle cell membrane). A cross-sectional view reveals the muscle fiber’s interior structure, showing numerous circular myofibrils packed within the sarcoplasm. The lower portion of the diagram provides two magnified views of the synaptic mechanism. The left panel shows the synaptic end bulb containing multiple synaptic vesicles filled with acetylcholine (ACh), positioned above synaptic clefts in the motor end-plate of the muscle fiber. A red arrow indicates the nerve impulse (action potential) traveling down the axon. The right panel, connected by a gray arrow labeled “Synaptic vesicle releases ACh by exocytosis,” depicts the release process in detail. Here, synaptic vesicles (shown in yellow with pink dots representing ACh) fuse with the presynaptic membrane and release acetylcholine into the synaptic cleft. The released ACh molecules (pink dots) then bind to ACh receptors (shown as gray structures) on the motor end-plate, which opens channels allowing sodium ions to enter, ultimately triggering muscle contraction. [Return to Figure 8.3.1]
Figure 8.3.2. This anatomical diagram illustrates the internal organizational structure of a skeletal muscle fiber with the sarcolemma (muscle cell membrane) partially cut away to reveal the underlying components. The sarcolemma appears as a beige-colored outer layer with several circular openings visible on its surface. Beneath the sarcolemma, the diagram shows three cylindrical myofibrils arranged in parallel, displaying their characteristic banding pattern in alternating red and yellow stripes representing the arrangement of contractile proteins. Surrounding and wrapping around these myofibrils is an extensive network of sarcoplasmic reticulum, depicted in yellow, which forms an elaborate tubular mesh structure. The terminal cisternae, which are enlarged portions of the sarcoplasmic reticulum, appear as cream-colored swellings positioned at regular intervals along each myofibril. Running perpendicular to the myofibrils are the T-tubules (transverse tubules), shown as tubular invaginations that extend inward from the sarcolemma. Where a T-tubule encounters terminal cisternae on either side of it, these three structures together form a triad, which is specifically labeled in the diagram. This arrangement is crucial for coordinating muscle contraction throughout the fiber. [Return to Figure 8.3.2]
Figure 8.3.3. This comprehensive diagram illustrates the complete sequence of events in skeletal muscle contraction, presented as a vertical flow chart. At the top, a motor neuron with its myelin sheath approaches a muscle fiber, and the text indicates “An action potential arrives at neuromuscular junction.” The neuron terminal contains visible synaptic vesicles shown as circular structures within the nerve ending. A gray arrow leads to the next stage, showing the synaptic end bulb (colored in yellow-green) positioned above the motor end-plate. The label explains “ACh is released, binds to receptors, and opens sodium channels, leading to an action potential in sarcolemma.” Multiple ACh receptors are depicted as mushroom-shaped structures on the muscle membrane surface, with the word “Excitation” indicating the resulting depolarization. The diagram then shows how the action potential travels along the T-tubules, which are invaginations of the sarcolemma extending into the muscle fiber interior. Below this, the sarcoplasmic reticulum (shown in tan/beige) releases calcium ions, illustrated as small circles flowing downward through red arrows into the cytoplasm. These calcium ions interact with troponin molecules on the thin filaments, shown in green with orange regulatory proteins. A myosin head (depicted in purple) is shown with ADP and Pi (phosphate) attached, ready to bind to the actin filament. The subsequent panel displays the interaction between thick filaments (shown in purple horizontal stripes) and thin filaments, labeled “Thick and thin filament interaction leads to muscle contraction.” A red arrow points downward to a cylindrical muscle fiber showing the characteristic striated pattern and cross-sectional view of myofibrils. Black arrows on either side indicate the direction of force generation. The final image at the bottom shows the muscle fiber shortened and bulging in the middle, with black arrows pointing inward from both sides, accompanied by the text “Muscle shortens and produces tension.” This demonstrates the ultimate result of the contraction cycle where the sarcomeres shorten, causing the entire muscle fiber to contract and generate force. [Return to Figure 8.3.3]
Figure 8.3.4. This detailed diagram presents the cross-bridge cycle of muscle contraction in five sequential stages labeled (a) through (e). Each stage shows the interaction between thin filaments (containing actin in green, wrapped by orange tropomyosin, with small circular calcium ions scattered around) and thick filaments (composed of myosin, shown in purple stripes). In panel (a), the thin filament displays actin subunits as green spheres with calcium ions bound to troponin complexes on the tropomyosin strand. A myosin head extends from the thick filament below, labeled as a crossbridge, with ADP and Pi (inorganic phosphate) molecules shown in yellow attached to it. Panel (b) shows the myosin head bound to the actin filament, forming the crossbridge in its high-energy configuration. A large red arrow points downward between panels, indicating progression to the next step. In panel (c), labeled “Power stroke,” the myosin head pivots, pulling the thin filament toward the center of the sarcomere (indicated by black arrows showing filament movement). During this power stroke, ADP and Pi are released from the myosin head. Another red arrow leads to panel (d), where ATP (shown in yellow with a starburst pattern) binds to the myosin head, causing it to detach from the actin filament. The label states “ATP attaches to myosin and myosin head detaches.” The final panel (e) shows the myosin head returning to its original cocked position after ATP hydrolysis, with the text “Cocking of myosin head.” The myosin head now has ADP and Pi bound again, ready to begin another cycle. Calcium ions remain bound to the thin filament throughout the cycle, keeping the binding sites on actin exposed for continued contraction. [Return to Figure 8.3.4]
Figure 8.3.5. This figure contains three diagrams (a, b, and c) illustrating different energy metabolism pathways in muscle tissue. Diagram (a) shows the creatine phosphate system, with “Resting muscle” labeled on the left and “Active muscle” on the right. ATP and creatine combine through the enzyme creatine kinase to form creatine phosphate (shown in a starburst shape) and ADP during rest. During muscle activity, this reaction reverses: creatine phosphate and ADP are converted back to creatine and ATP, which provides energy for muscle contraction. Diagram (b) depicts glycolysis, showing how blood glucose and muscle glycogen are converted to glucose, which enters the glycolysis pathway enclosed in a box. This process produces two ATP molecules and two pyruvate molecules. The diagram indicates two possible fates for pyruvate: if oxygen is present, pyruvate enters aerobic respiration; if no oxygen is available, pyruvate is converted to lactic acid that enters the bloodstream. Diagram (c) illustrates cellular respiration in mitochondria, represented by a pink oval organelle. Three fuel sources (blood glucose, pyruvic acid, and fatty acids) enter the mitochondrion along with oxygen. Inside the mitochondrion, cellular respiration occurs, producing a large cluster of ATP molecules (shown as multiple starburst shapes), along with heat, carbon dioxide, and water as byproducts. These three diagrams together demonstrate the complete energy metabolism system that muscles use during both rest and activity. [Return to Figure 8.3.5]
Figure 8.3.6. This graph illustrates oxygen consumption patterns across three phases: rest, exercise, and recovery. The vertical axis represents oxygen consumption, while the horizontal axis shows time progression. At rest, oxygen consumption remains at a baseline level labeled “Resting O₂ consumption.” When exercise begins (indicated by a light green shaded region), oxygen consumption rises sharply, creating a dark green triangular area labeled “O₂ Debt” at the start of exercise. This represents the lag between the onset of exercise and when oxygen delivery meets demand. The oxygen consumption then plateaus at an elevated level during the “Steady state” period, shown as a lighter green rectangular area labeled “O₂ requirement,” where oxygen supply matches the metabolic demands of exercise. A horizontal dotted line marks this steady-state level. After exercise ends and the recovery phase begins, oxygen consumption gradually decreases but remains elevated above resting levels for a period, creating another dark green shaded area labeled “EPOC” (Excess Post-exercise Oxygen Consumption). This represents the elevated oxygen consumption needed to restore the body to its pre-exercise state. Eventually, oxygen consumption returns to the baseline resting level, completing the cycle. [Return to Figure 8.3.6]
Report an Error
Did you find an error, typo, broken link, or other problem in the text? Please follow this link to the error reporting form to submit an error report to the authors.
